Title: Notes Upon Indigo
Author: John L. Hayes
Release date: May 3, 2020 [eBook #62008]
Language: English
Credits: Produced by Ronald Grenier. (This file was produced from
images generously made available by The Internet Archive)
Transcriber’s Note:
The cover image was created by the transcriber and is placed in the public domain.
PART I.
3
NOTES UPON INDIGO.
A publication devoted to the interests of the woollen manufacture, while giving due prominence to its first raw material, wool, cannot neglect the secondary materials which enter into finished fabrics. The attractiveness and utility of the largest class of these fabrics are due to the hue given them by the dyer; and of all the coloring materials one of the most precious is indigo. In former times, as it still does at the East, it occupied with madder the place of one of the two most important of all dyeing materials. Forced of late years to give way to the marvellous products of modern chemistry, it will doubtless resume its place under the influence of a more enlightened economy and a more subdued taste. To contribute to the hastening of this return is one object of this essay. The most usual reproach against American fabrics is the want of stability in our dyes,—a reproach without justice, if applied to American fabrics alone; for the cheapening of dyestuffs is practised in all the so-called manufacturing nations, and is contemned alone in the East, from which we have derived our arts, and by the people whom we despise as barbarous. To remove this reproach from American fabrics would be worthy of no little temporary sacrifice on the part of our manufacturers.
The value of indigo as a dyeing material is due to the great stability of the blue color, and the derivatives from blue, which it gives to fabrics, especially of wool and cotton. It is not sufficient that a dyed fabric should preserve its color when 4 submitted to violent tests, as when acted upon by vegetable or mineral acids or alkaline or soapy baths: the only stable dyes are those which resist air and light, the two destructive agents of vegetable colors. Indigo, from the remarkable manner in which its color becomes fixed upon a fabric, to be hereafter explained, possesses properties of resistance and stability in a higher degree than any blue dye. And when we consider that this blue has not only its own hue, but is the best foundation for blacks, greens, purples, and even browns, the importance of these properties cannot be over-estimated. Says M. de Kæppelin, a chemist and manufacturer of Mulhouse, in one of a series of articles furnished to the Annales du gênie Civil, 1864: “So high are the properties of resistance and stability which indigo possesses, that it is perhaps to be regretted for the art of the dyer and manufacturer of printed calicoes, that the use of indigo becomes more and more rare, and that the recent discoveries which modern science has placed at the service of industry are daily eliminating it from our factories. I have observed that whenever we have to dye stuffs of a high price, it is indigo which always serves as a base for the foundation of all the blue colors, or of those which are derived from blue. It is the same for the fabrication of printed tissues, which serve for the poorer classes, whose colors should have great stability without much increase of cost. But of late years, especially, we find a tendency to employ colors of little stability, and to prefer them, even in the class of fabrics first referred to, to those which are more fast, on account of their vivacity and freshness of tone. It is this tendency, which the consumer partakes of even while complaining of it, that the textile manufacturers ought to seek to combat. How often have I heard the greatest manufacturers of Alsace deplore the obligation which they felt that they were under of printing their tissues by means of colors so fugacious and so little resistant as those composed from aniline. We must hope, then, in the interest of that industry, that while adopting the marvellous discoveries which science is every day making, there shall be made a less general application of them, and that we shall 5 return to the fabrication of the styles which necessitate the more constant employment of coloring materials,—less brilliant, it is true, but more adherent to the tissues, and less alterable by air and light. It seems to me, also, that taste would lose nothing; and that printed stuffs, colored in a manner less brilliant, but more harmonious, would be perhaps more appreciated, especially by those who use them.”
The tendency to substitute the brilliant for the stable dyes prevails too much in our own manufacture. A very considerable cloth manufacturer replied to our inquiry as to the extent to which he used indigo: “I hardly use it at all; the dye of the indigo blue is not bright enough to be popular.” On the other hand, we have heard our leading manufacturer of carpets, whose cultivated taste has led him to partake of M. de Kæppelin’s views, deplore the introduction of aniline dyes, as a positive calamity to the textile industry. It is the influence of the trade, the immediate consumers of fabrics, rather than the judgment of manufacturers, which promotes the use of the modern fugacious dyes. The dealers desire not only to imitate the fashionable colors of European goods, but to secure the utmost cheapness. One of our largest manufacturers of woollen goods, who had made a special study of the best processes abroad, and was desirous of bringing better dyed goods into more general consumption, urged one of his largest customers, an extensive dealer, to allow him to dye the waterproof cloakings which he was furnishing for his house, in fast indigo colors, assuring him that he would charge simply the additional cost of the indigo, without profit. The offer, which involved the cost of only a few cents a yard, which would have been gladly paid by the last consumer if the difference of value had been made known, was declined. It is not improbable that the inferior goods which the manufacturer was compelled to furnish were sold to the public as fast dyed. Our manufacturers, therefore, may not have been responsible for the predicament in which the most enthusiastic defender of our protective policy found himself, as we have it from his own lips. Being about to make a speech in Congress in defence of American industries, he put on, for the 6 first time, a coat declared to have been made of American cloth. Sitting down, heated and perspiring from the excitement of his effort, he found that beneath the arms whose gestures had enforced his eulogies of American industry, the pretended fast blue of his coat had become red, literally blushing for its unmerited praise. That fast-dyed goods of the highest excellence can be and are furnished by American manufacturers, is shown by our army cloths. The government specifications, copies of which are published elsewhere in this number, require that all the blue woollen cloth, cap cloth, and flannels furnished for the army shall be “pure indigo dyed.” The requisition is strictly enforced. The admirable effect of this regulation may be witnessed at any dress parade of a battalion of United States soldiers. The persistency and uniformity of the hue under constant wear—the cloth of the common soldier in its superior dye often favorably contrasting with the finer but fancy dyed cloth of the officer—is one of the circumstances which justify the assertion, that our army is the best clothed in the world. The contrast is more remarkable still with the quondam blue cloth, converted by sun and rain-into every shade of shabbiness, which we purchased in Europe for our soldiers at the commencement of our late war.
Indigo is a coloring material of vegetable origin, which owes its color and its important applications to a direct blue principle, known under the name of indigotine. It has been used as a dyestuff from time immemorial, by the inhabitants of India; and it is from the East, the cradle of the textile arts, that Europe has derived it. It was probably received from India by the Greeks, among other products first made known to them by the expeditions of Alexander the Great. Dioscorides clearly refers to indigo in mentioning the two coloring matters brought from India. Pliny mentions a coloring material, having an admirable mixture of blue and purple, as coming from India, which he calls indicum. That he refers to indigo is curiously manifest by the test which he gives, by which the genuine drug might always and 7 certainly be distinguished from the spurious. This is by putting it on live coals, when, says he, “the true indicum will burn with a flame of a most beautiful purple tint.” The purple vapor from burning indigo is still a characteristic test. The Romans, it is apparent, used indigo only as a pigment, not knowing what is still the most important art connected with its use,—how to make it soluble so as to be available in dyeing.
That indigo as a commercial product was first obtained from India is not only proved by the testimony of Pliny, and other ancient writers, but is confirmed by a variety of circumstances, and particularly by its name, which is known to have been nil in the Hindu language, from the earliest times of which there is mention of it. This name is still given by the Hindoos to the color blue, and to all the plants producing indigo. The Arabs and Egyptians, who obtained a knowledge of indigo from India, adopted the Hindu name, the Arabs calling it nil or nir, and the Egyptians nil or niel. The Portuguese preserved the Indian name, with a slight modification, the substance being called aniliera in their language. The coloring substances afterwards found in coal-tar having been first found in indigo, modern science has adopted for them the name of aniline.
It has been asserted that this substance was not known in Europe until the time of the discovery of the passage to India round the Cape of Good Hope. But Dr. Bancroft has shown that indigo was brought by merchants from India to Alexandria, and thence to Venice, when that city was the entrepôt of Europe and the East. It doubtless contributed to the excellence which the Italian states first attained in the wool manufacture. The drug was called endigo in Venice, and it is from that city that we have derived its name and use. It was imperfectly known in England under its Spanish name in the sixteenth century, for we find in Hackluyt “Voyages” his instructions to a traveller who was going to Turkey to ascertain “if anile, that coloureth blue, be a natural commodity of those parts, and if it be composed of an herbe.”
The general introduction of indigo into Europe was impeded by legislative enactments, prompted mainly by those employed 8 in industries which it threatened to displace. These were chiefly the producers of and dealers in woad, formerly used exclusively for dyeing blue, and the corporation of woad dyers. When dyers from Italy and Flanders attempted to introduce the superior dyes of indigo, the woad interests were sufficiently powerful to induce the Elector of Saxony to denounce the use of the new dyestuff. It was pronounced in the Diet of the Empire as “a corrosive color,” and “fit food only for the devil,” fressende teufels. Similar propositions were made in England and France, in which latter the free use of indigo was not permitted until 1737.
Although indigo as known in the arts is a product of vegetable origin, we must not omit to notice that one source of its production is the human body. It was discovered some years since that the blue color sometimes found in diseased urines, and in certain suppurations, is due to indigo. Dr. Schunck, in some papers read before the Royal Society, has shown that it is a frequent constituent of urine secreted by persons in a healthy state, and that, in fact, it is produced generally when persons do not take sufficient exercise; and he has several times succeeded in producing it by taking in his food a rather large excess of sugar. He has found this substance also in the urine of beef cattle. It must also be observed that the chemical actions of indigotine with oxidizing agents, showing indigo to have a very close relation to aniline and carbolic acid, both products derived from coal-tar, have produced in the minds of chemists the conviction that indigotine, like alizarine, the coloring principle of madder, will one day be artificially produced from coal-tar.
The plants which are known to furnish indigo are quite numerous, being not less than sixty; they do not all belong to the same family, and none of them contain the coloring principle already formed. The most important belong to the leguminous family, from which most of the vegetable dyes are derived, and to the genus indigofera. The species cultivated and most esteemed are Indigofera tinctoria, I. disperma, I. anil, I. argentea.
9

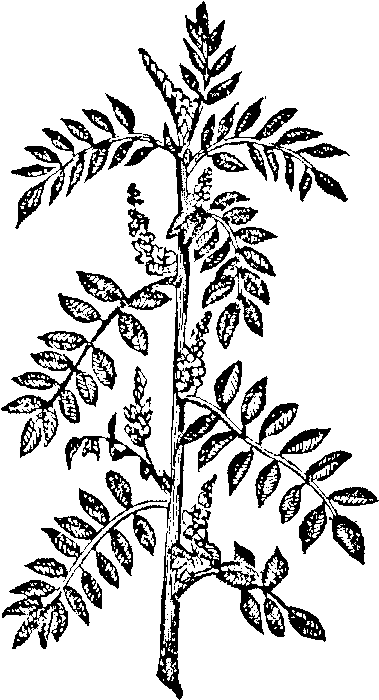
The principal source of the indigo of commerce is the Indigofera tinctoria. The accompanying figure is a correct representation of the plant, and we may dispense with a description of its botanical characters, observing only that the plant has a half woody stem, and rises to the height of from three to five feet. The plants exhale a strong odor towards evening in the fields where they are cultivated. The leaves have a disagreeable taste, and rapidly putrefy in water. The plant originated in Campaja, or Guzerat, but is cultivated in Hindostan, China, Java, and in the East Indies generally. It was carried by the Spaniards to South America and the West Indies, and it can be acclimated in all hot countries. The Indigofera argentea, or indigo plant of Egypt, furnishes the indigo produced in that country and Arabia.
The culture of the plant and the production of commercial indigo is carried on a vast scale in Lower Bengal. We have before us a large map, placed at our disposal by an India merchant of Boston, showing the location of each of the hundreds of factories of that important centre of production. These factories have been developed by British enterprise; and India thus receives some slight compensation for the ruin of her cotton manufacture by the same influence.
The propagation of the indigo plant in that country is made by sowing in a thoroughly tilled silico-argillaceous soil. The seed of the plant is sowed annually in the spring or autumn, 10 according to the variety used, some germinating more slowly, and requiring to remain in the ground longer than others. The time of putting in the seed is also governed by the nature of the soil and its position in respect to neighboring rivers. In the lowlands subject to inundations, the indigo ought to be all cut at the period of the rains and inundations, which would destroy the crop in a brief time. Besides, during the rainy period the planter has at his disposal sufficient water to commence his operations of fabricating the indigo, which is the suitable time for beginning the cutting of the plant. The time of cutting the indigo plants is therefore regulated by the elevation of the land and danger from floods. The high lands are always sowed several weeks after those subject to inundations.
The Chinese prick out the young plants in parallel rows, always preserving the land quite clear of weeds. By taking away the blossoms of the plant before their development, they increase the growth of the leaves, and, consequently, the return of indigo; for it is in the leaves principally that the coloring material is found.
In certain localities the planters break off the leaves which have acquired a bluish green tint. But more frequently the whole plant is cut down close to the ground in the months of June or July, when the flowers begin to open. The portion of the plant which remains pushes up quite rapidly, and furnishes a second, and even third, and sometimes, though rarely, a fourth cutting. The quality of the product diminishes according to the number of the cuttings.
The plant called nil, cut down to the root and gathered up in packages, is worked up the same evening. The package is formed from the product of a space of land embraced by a chain about three yards long. The value of the first material changes with the value of the soil. Thus, one soil produces a plant which has many stems and few leaves, while another gives many leaves and few stems. The richness in coloring material depends upon the quantity of leaves, but varies also with an equal weight of leaves with atmospheric influences. Thus regular dealers in the article observe a marked difference in the quality of indigo in different seasons.
11
M. A. Koechlin Schwartz has recently published some interesting notes upon the preparation of indigo in Lower Bengal. In that country, which furnishes excellent indigo, the factory includes, besides filters, presses, a steam-engine, drying apparatus, and reservoir of water, two lines of vats, arranged one above the other, from fifteen to twenty in each line. These vats are built up with bricks, and covered with a strong coat of solid and well made stucco. They are square, about six yards on a side, and about a yard deep. The back row is about a yard above the front one. The plant is fermented in the vats of the upper row; when the operation of fermentation is terminated, a faucet is opened, and the liquid is run into the lower vat. The water of the Ganges, which is relatively pure, and thus well suited for this work, is brought into basins of deposition, where it becomes clarified, and is distributed by a common canal to the vats of the upper row. The plants, cut in the morning and bound up into packages, come to the factory after midday, and are thrown into the vat in the evening. A vat contains one hundred packages carefully arranged, one beside the other; heavy timbers are placed upon the plants, which are pressed down by means of large wedges. It is necessary that the plants should be pressed together very compactly, as without this the fermentation does not take place to advantage. At nightfall the water is introduced into the vats, and fills them so as completely to submerge the plants. The fermentation is more or less prolonged according to the temperature. Its duration varies from nine to fourteen hours. The workmen judge as to the procedure of the operation by withdrawing a little of the liquid in the lower vat. If it is of a clear pale yellow when withdrawn, it will furnish a product less abundant but more pure than if of a deep gold color.
At the moment of its issue from the fermenting vat the liquid is of a yellow color, more or less deep. The liquid is allowed to remain undisturbed for a brief period, when twelve naked men, armed with long bamboos, enter the vat to beat the water while it is still warm. During this time the upper vat is emptied and cleaned out for the succeeding operation. One vat requires 12 seventeen workpeople (twelve men and five women). They thrash the water for two or three hours. The liquid passes by little and little to a pale green, and the indigo is found on suspension in the form of small floccules. The liquor is suffered to remain undisturbed for half an hour; it is then gradually decanted by opening, one after the other, the discharging holes placed at different heights. The water returns to the river, and the precipitate, under the form of a thin bouille, is turned into a reservoir. This bouille is pumped up into a vessel, and made to boil for a moment to prevent a second fermentation, which would injure the quality of the product, by turning it black. It is suffered to rest about twenty hours, and the next morning it is again subjected to boiling, the ebullition being kept up three or four hours. The boiling deposit is then turned off upon a large filter, through which the water drips. This filter is composed of a vat constructed of masonry, covered with stucco, about eighteen feet long by six feet wide and three feet deep. This is covered with bamboos, upon which is a grating of smaller reeds, and above by a stout strained cloth. There remains upon the cloth a thick paste, of a deep blue and nearly black color. The water which is run into the vat deposits some indigo which has pressed through the filter. This is decanted after being allowed to rest, and the turbid liquid is boiled the next day with the fresh indigo.
The paste of the filter is introduced into some small boxes of wood, pierced with holes, and provided above and below with a strong cotton cloth. The whole is again covered with a piece of stuff, and then with a covering of wood, pierced with small holes, and it is placed under a press, the force being gradually applied, so as to cause the water to run out as much as possible. There is withdrawn from the box a cake of the size of a cake of Marseilles soap. The water squeezed out flows back into the filtering vat, to be boiled again with the fresh indigo. The drying of the cakes ought to be done very slowly.
The dry-house is a large building of masonry, quite high, and pierced with many openings, provided with narrow blinds, to prevent the direct light of the sun from penetrating into the 13 interior. Care is taken also to surround the dry-house with large shade-trees. The cakes take from three to four days to dry, after which they are packed in small boxes and carried to Calcutta, the great market of Bengal.
The details above given apply to the factories managed by European planters. The natives operate in nearly the same manner, but with less care, and consequently their products are inferior. The average product of indigo in Lower Bengal is stated at 4,000,000 kilograms, or 8,840,000 pounds per year. The most remarkable fact to be noticed in these operations is, that the blue principle is developed by chemical action from certain absolutely colorless principles existing in the plant. The theory of the change effected is still somewhat in doubt, because no chemist has studied the fresh plant, and observed upon the spot the phases of the operation of the production of indigo on a large scale. But the most accepted theory is that derived from the researches of Dr. Schunck, upon the isatis or woad-plant, which produces indigotine in a much less degree than the true indigo plants; viz., that the indigo exists in the plants combined with sugar, forming a glucoside, to which he gives the name indican. This compound, under the influence of fermentation in the manufacturing process, is supposed to be unfolded into indigo and sugar.
Without dwelling upon this question, which is beyond our province, we observe that the plants of the genus indigofera are used for the production of commercial indigo, on account of the greater richness in the coloring principle. Other plants, which furnish the same coloring principle, indigotine, are more frequently used directly in dyeing to furnish the blue principle than they are for the production of indigo.
The most important of these plants, although there are others, such as the Polygonum tinctorium and the Nerium tinctorium, is the Isatis tinctoria, which produces pastel, or woad. This, plant belongs to the family of cruciferæ, and is a biennial. It is represented in the accompanying figure.
14
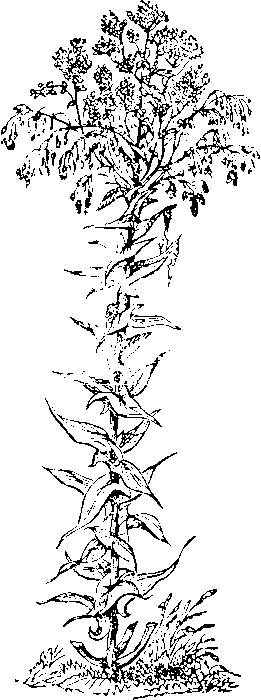
The leaves which surround the stem are collected in May or June of the second year, when they begin to turn yellow. The wasted and dried leaves are sometimes used directly for dyeing, but more generally the leaves, after being cut and dried, are carried to a mill, and then ground to a paste, after which it is formed into a mass or heap, and being covered to protect it from rain, is left to undergo a partial fermentation for about a fortnight. The heap is then well mixed and formed into balls, which are exposed to the sun and wind to dry, and thereby prevent the putrefaction which would otherwise take place. Being afterwards collected in heaps, these balls again ferment, become hot, and emit the odor of ammonia, which Hume tells us, in the History of England, gave such offence to Queen Elizabeth that she issued an edict to prohibit the cultivation of this plant. After the heat has continued for some time, these balls fall into a dry powder, in which form the woad is usually sold to the dyer. The best French woad comes from Provence, Languedoc, and Normandy. In Germany, the pastel of Thuringia is used almost exclusively; 15 the packages have the trade-mark of three towers, with the numbers 4, 5. In this country, owing probably to the prejudices of practical dyers, who have generally come from England, the Lancashire woad is almost exclusively used. The very little imported of late years, ranging from two thousand to twelve thousand dollars annually in value, is used for mixing with indigo in the so-called woad vat, to be hereafter described.
The following description of the indigoes of commerce is taken principally from Schutzenberger’s excellent treatise on coloring materials. It coincides very nearly with that given by Napier from Dumas and Chevrueil. Indigoes are classed, according to their origin, into three groups.
1. Indigoes of Asia (from Bengal, Oude or Coromandel, Manilla, Madras, and Java).
2. Indigoes of Africa (Egypt, Island of France, Senegal).
3. Indigoes of America (Guatemala, Caraccas, Mexico, Brazil, and the West Indies).
The three varieties in most esteem are those of Bengal, Java, and Guatemala.
Indigoes of Java.—These are distinguished by the great purity of their coloring material. They contain the minimum of extractive organic matter. If, in spite of this, they do not give a high yield of indigotine; this is owing to a mixture of silicious mineral substances with their paste. The paste is soft. It adheres strongly to the tongue, and its density is feeble. They are generally of a pure blue, light or ash colored in the kinds which are less rich, and of a magnificent violet blue in the superior qualities. The last take a beautiful copper color when scratched by the nail. They are placed in the very first rank among all indigoes in respect to fineness and beauty, if not in richness in the blue coloring principle. Their purity, complete absence from carbonate of lime, and the small quantity of foreign organic materials which they contain, cause them to be much sought for, for the preparation of carmine of indigo. The consumption of the Javan indigoes in this country is so small as not to be appreciated.
16
Bengal Indigoes.—These are the indigoes par excellence, for in them are found the most varied qualities, from the most beautiful and rich to the most ordinary. The superior qualities are of a deep violet blue, with a fine and uniform paste; they adhere to the tongue, are easily pulverized, and take a beautiful coppery tint when scratched by the nail. The fresh fracture shows a magnificent purplish blue reflection. Their yield in indigotine does not surpass seventy-two per cent.
After these come the reddish-violet indigoes with a purplish hue, and a fracture more uniform and shiny. They are also more dense and hard than the superior qualities. The reddish hue does not proceed from the greater or less amount of coloring material contained, but from the presence of a greater quantity of brown and red extractive matter. These qualities are not to be despised, for the kinds which give the best results in the dyeing vat are found in these indigoes. It would seem, in fact, says the author whom we are following, “that the browns and reds of indigo play an important part in vat dyeing, that they are able to become dissolved and to fix themselves upon the tissues at the same time as the indigotine, and thus operate to reinforce the hue. The fact is, that dyers generally prefer the reddish indigoes to the other varieties.” Among the Bengal indigoes there is found a clear blue variety, less rich in coloring matter, but also more exempt from organic substances. The impurity is constituted by mineral matters. It is less dense, adheres strongly to the tongue, and does not take a coppery hue, like the other varieties, when scratched by the nail.
The worst qualities of the Bengal indigoes, as in all the species, are the clear blues, shading on to gray or green. This coloration denotes a great quantity of extractive matter different from the indigo brown which characterizes the red varieties, and completely inert. These indigoes are hard, dense, adhere little or none to the tongue, and do not show coppery reflections when scratched.
The most skilful connoisseurs distinguish forty-three varieties of Bengal indigo. The most important are the following:—
1. Superfine blue, light or floating.—Color bright blue; 17 light, friable, and spongy; adherent to the tongue, soft to the touch, showing coppery reflections when rubbed by the nail; paste uniform and pure.
2. Fine blue.—Like the preceding, but the color a little less vivid.
3. Violet blue.—A little less light and friable. Has a violet blue.
4. Superfine violet.
5. Superfine purple.
6. Fine violet.
7. Good violet.
8. Red violet.
9. Ordinary violet.
10. Good soft red.
11. Good red.
12. The indigoes, fine coppery, good coppery, ordinary coppery, and low coppery.
The Indigoes of Oude and Coromandel.—These are made in the interior of Hindostan. Those of the best quality correspond to the middling Bengal indigoes, and are met with in square masses, having an even fracture, but are more difficult to break; the inferior qualities are heavy, of a sandy feel, having a blue color, bordering on green or gray, or even black; often in large squares, and covered with a slight crust or rind of a greenish color. They are the most difficult to break of all the indigoes of commerce.
Madras Indigoes.—They have a grained fracture, and are of a cubical figure. The superior qualities have no rind. The qualities are fine blue, mixed violet blue, and ordinary. They are all lighter, and less rich in coloring matter than the Bengal indigoes.
Manilla Indigoes.—These occur in cubical blocks, flat squares, or in irregular pieces. They are light, with a fine paste, and of a clear blue. They effervesce with acids, showing the presence of carbonate of lime incorporated in their paste. They are consequently poor in coloring material, and are hence almost exclusively used as a bluing material in washing fabrics.
18
American Indigoes. Guatemala.—These indigoes are produced now altogether in Hunduras, although they still retain in commerce the name of Guatemalan. They are generally found in small pieces, irregular in form and size, and come in envelopes of skin containing about half as much as the Bengal chests. Putting aside the difference in exterior form, these indigoes approach very closely to those of Bengal. The same qualities are found, only they are more frequently mixed. The clear blue is more rare, and, when it is found, it is poorer in coloring matter. In purchasing these indigoes it is necessary to beware of the reds, which often contain a strong proportion of the brown extractive matter. It is not rare to find among the Guatemalan indigoes beautiful specimens of the blue violet, equal to the richest Bengal variety. Unfortunately, this superior variety is generally mixed with inferior kinds, as to have less value. The American indigoes are classified as follows:—
Guatemala floro.—Bright blue, paste uniform, soft and light. This variety, in Bancroft’s time, was the most esteemed of all indigoes.
Guatemala sobresaliente.—Less light, the paste firmer and the blue less beautiful.
Guatemala corte, or copper-colored.—Paste less firm and heavier, coppery red.
Caraccas.—These resemble very much the Guatemala varieties. The qualities are designated by analogous names, but they are, in general, less esteemed than the preceding.
Mexican.—They hold an intermediary rank between the Caraccas and Mexican.
Brazil.—These indigoes are in small rectangular parallele-piped masses, or in irregular lumps of a greenish gray color externally, and having a smooth fracture, a firm consistency, and a copper-colored tint of greater or less brilliancy.
The indigoes of Africa and Egypt.—These have only been manufactured within the last twenty years; they are in flat squares. The paste is fine and quite light, and the color pure blue or bordering on violet. The varieties are distinguished as fine blue and good violet and red.
19
Indigoes of the Isle of France and Senegal. Rare in commerce, but of good quality.
The indigoes of the inferior qualities, characterized by a salt-like color, bordering more or less upon green; by a coarse, uneven, and very dense paste; by not adhering to the tongue, and by not showing a coppery color when scratched,—can never be employed to advantage, notwithstanding their low price. The purchaser of these qualities must be guided solely by the results of analysis; for an article is found in commerce whose richness in indigotine does not exceed twelve to fourteen per cent. The presence of so high a proportion of foreign matter prevents the chemical change which the indigo ought to undergo in the dyeing vat; and this foreign matter, added to the deposits of the dyeing vats, causes great loss of the coloring matter. These indigoes should be used as little as possible, especially in the cold vats used for dyeing cotton and linen. The middle varieties of the Bengal and Guatemala indigoes, and, above all, the red varieties, produce in the cold vats the most advantageous results. The lower qualities above spoken of present less inconvenience in the hot vats used for dyeing wool; and it is for this purpose that they are generally used. In considering the previous observations, the wool manufacturer may arrive at this conclusion: that while he can, with less loss than the maker of cotton fabrics, make use of the lowest qualities of indigo, he will obtain the best results from the middle qualities of the reddish Bengal indigoes.
The skilled dealers in indigo recognize not only the above distinctions, founded upon the country of production, color, and physical qualities, but they observe whether the article has any of the following defects, which are designated by certain well-understood terms: such as whether the indigo is sandy,—when brilliant points are observed in the interior, which are in reality particles of sand; spotted, that is to say, of unequal tint, and marked by small blackish points; ribboned, marked by transversal bands of a paler, and sometimes red color; burnt, the pieces having a scorched appearance, due to rapid drying, and 20 separating into small black fragments under the pressure of the hand; crumbly, when in pieces of irregular figure, proceeding from fractures of the squares; cold, when the indigo does not adhere to the tongue. The above classification is presented with a full knowledge that these distinctions are by no means recognized in the ordinary commerce in this article. It is not, however, without interest as an illustration of the minute attention given to this subject in Europe, where a higher manufacture requires a nicer investigation of the qualities of materials employed.
It is evident that the commercial form and the high price of this drug favor fraud, and the desire to illicitly introduce foreign substances into the paste. It is important, therefore, that the purchaser should carefully ascertain the actual value of the article which he is to use. He should know not only the proportion of indigotine contained, which varies in the commercial indigoes from twelve to seventy-five per cent, but the hardness and density. A good indigo ought to have qualities which can be recognized by the eye and touch alone. The first and the only examination ordinarily made by purchasers is in respect to the physical qualities of the article. Different pieces are selected, and their fresh fracture is attentively observed. The purchaser observes whether the squares are like each other, and if the parts of the same piece present the same tint. He determines the porosity by the simple means of applying his tongue to the fresh fracture. The more rapid the adherence of the tongue, the more porous the indigo. By scratching the piece with his finger-nail, he determines the extent of the coppery reflection, — an important test.
From all these characters, taken together, the purchaser can form quite a correct idea of the value of indigoes in general; and the greater number of dyers, both in Europe and this country, are satisfied to make their purchases with only this physical examination. The most experienced dealers in this country 21 make no other examination than the physical one. An eminent indigo broker in Boston has permitted me to copy the following memoranda for the physical examination of indigo from his notebook.
The chief signs of good indigo are its lightness, feeling dry when touched, and, when broken, appearing of a beautiful violet blue. Good indigo swims in water; if thrown upon burning coals it emits a violet-colored smoke, and leaves but little ashes.
In selecting indigo the large regularly formed cakes should be preferred,—those of a fine, rich blue color, extremely free from the white adhesive mould, [1] and of a clean, neat shape. When broken, it should be of a bright purple cast, of a close and compact texture, free from specks or sand, and when rubbed with the nail should have a beautiful shiny coppery appearance; when burnt in a candle it should fly like dust; that which is heavy and dull colored should be rejected. Indigo is estimated and classed in commercial language, as follows: fine blue, ordinary blue, fine purple, inferior purple, and violet, strong copper, and ordinary copper. It is purchased by the factory maund (74⅔ lbs. The Bazaar maund is 82²⁄₅₀ lbs.), packed in cases containing on an average 2¼ cwt., dammered (pitched) and covered with gunny bagging.
Still, in making large purchases, as a measure of wise precaution the chemical test should be added. This is used to ascertain the proportion per cent of indigotine which a given indigo has. The determination of the quality of indigotine contained is not alone sufficient to fix the value of an indigo. With an equal yield of indigotine, the indigoes are always to be preferred which have a light and soft paste; and for the preparation of the indigo vat the preference should be always given to the violet red rather than to the clear blue indigoes.
The chemical works which treat of this subject give elaborate details of a great number of processes for determining by chemical tests the amount of indigotine, or the coloring material in indigoes. To give these numerous processes would only 22 confuse the reader. In our own confusion upon this subject we submitted the descriptions of these various processes to one of the most eminent and practical of American chemists, Dr. Charles T. Jackson, an official State Assayer for the State of Massachusetts, who has had much experience in testing indigo, with a request that he would describe the process which he approves and practises. He has obliged us by the following communication:—
Boston, Nov. 21, 1872.
No. 47 Court Street, Room 4.
John L. Hayes, Esq.
Dear Sir,—In reply to your inquiry as to the simplest method of analyzing indigo, I would say that I first ascertain the amount per cent of earthy matters and metallic oxides, in the samples brought to me, by burning a weighed quantity in a counterpoised platinum crucible, until all organic matters are removed or consumed, and then weighing the ashes obtained. The ash is then subjected to analysis in the usual way, and lime, alumina, peroxide of iron, and some other earthy impurities are separated.
Then, to determine the amount of coloring matter, or indigotine, I make use of a standard sample of pure reduced indigo, which is dissolved in the most concentrated sulphuric acid, and diluted with water after solution. Then I ascertain how much bleaching powder (chloride of lime) is required to dissolve the solution. This is the quantity required for absolutely pure indigo.
Now, the indigo of commerce does not contain more than say from forty or fifty per cent of pure indigotine, and of course will require a smaller quantity of bleaching powder to decolor it; or the quantity of bleaching powder to decolor a given weight of pure indigo may be weighed out, and the sample to be compared having been dissolved in strong sulphuric acid, and diluted with water, is to be poured in and stirred or shaken well until the point of decoloration is ascertained. In this case it is best to weigh out at least twice as much of the sample to be tested as was used of pure indigo, and to measure the solution in a graduated glass vessel,—an alkalimeter, for example,—so that by measure we may know exactly how much of the sample we add to the solution of bleaching powder. Thus the relative coloring values of the samples may be readily ascertained.
If you have no purified indigo on hand, you can make a good 23 comparative trial of your samples against a perfectly good sample of Bengal indigo, which may be kept for a standard of comparison. Very useful practical results may thus be obtained.
It is well, however, to keep on hand a standard sample of pure indigo, prepared from reduced or white indigo, as directed by Berzelius (vol. vi. page 3, French ed., 1832), and in Muspratt’s Chemistry applied to the Arts (Dyeing, Indigo).
In the analysis by reduction of indigo, the process is simply as follows: Reduce the indigo to fine powder, and weigh it; weigh out an equal quantity of pure quicklime (made from pure white marble). Measure in a graduated vessel a certain volume of water. Slack the lime with a portion of this water. The rest of this water is to be used in rubbing up the indigo in a mortar. Then the slacked lime is to be mixed with the indigo, rubbing the substances well together. Introduce the whole into a large flask; 1½ to 2 litres (about 3 to 4½ pints) of water is required for 1 gramme (or about 15 grains of indigo). The flask and contents are then to be exposed to a heat of from 176° to 190° F. for some hours. This is best effected in a water bath. By this digestion the lime is made to combine with the indigo brown, and the coloring matter is set at liberty. Dissolve in the liquor a little protosulphate of iron, exempt from copper, and reduced to a fine powder. The flask is to be corked and well shaken, and allowed to cool. When the sediment is settled, decant the clear solution by means of a syphon into a graduated glass. The coloring matter oxidizes by exposure to the air; and to favor this oxidation and to keep the lime in solution, add muriatic acid to the liquor. When the liquor has become clear, filter and collect the precipitate on a weighed filter, which wash with hot water, and dry at a temperature of 212° F. Thus we can learn, by weighing the filter again, how much indigotine is contained in the sample.
If we make use of 200 measures of water, and have drawn off 50 measures of the solution to oxidate, and this 50 measures has produced 10 grains of indigo, the whole sample evidently contained 40 grains of indigo blue.
This method serves both for an assay of the sample and the production of a standard sample of pure indigotine. The operation may be carried on upon a larger scale for the manufacture of a standard sample.
Yours truly,
C. T. Jackson.
24
Dr. Jackson adds the following note:—
In the processes given I have not referred to the qualitative analysis or testing for all the kinds of adulterations, but have given only valuation of the coloring power of indigo.
I have had occasion to search indigo for Prussian blue, an occasional adulterant. This is ascertained by caustic potash, which becomes in part an oxide if Prussian blue is present. This acidulates with muriatic acid, and, tested with sulphate of iron, proves, by formation of Prussian blue, the presence of the ferrocyanide of potash in the solution, and hence Prussian blue in the indigo. Lime and clay are the usual adulterants, and oxide of iron is often present accidentally or from the clay adulterants. Starch and flour are rarely used, as they add little to the weight.
C. T. J.
Many experienced purchasers in this country pay no regard to this mould, as it weighs scarcely any thing.—Ed.
Before proceeding to a consideration of the practical applications of indigo in manufacturing, we must pause to make some general observations upon the commerce in indigo.
The first European impulse given to this commerce was made by the Spanish and Portuguese. They not only imported indigo from the Indies, but established its fabrication in their colonies. To them we owe its production in Guatemala, Caraccas, and Brazil. The French exported from the Island of San Domingo, only, in 1774, 2,350,000 pounds weight of this commodity. British influence was exerted in favor of the development of this article in the American colonies, and, in 1773, in the space of twelve months, over a million pounds of indigo were exported from South Carolina. The production in India was at that time of little importance. It was not until 1783 that the attention of the English was directed to the culture of indigo in India for European consumption, that produced by the natives being all consumed in their own manufactures. In the hands of the English this product rapidly rose to be the most important of India, in a commercial view, except that of rice. The small cost of a factory, and the comparatively small capital required for 25 this production, caused the indigo culture to be preferred to sugar planting. The importation and sale of this commodity at the East India House, in 1792, amounted to 581,827 lbs., while the importation into Great Britain from other parts of the world amounted to 1,285,927 lbs. In 1806 the importation from the East Indies, and sales at the East India House, amounted to 4,811,700 lbs., and produced in sterling money £1,685,275. In the year 1862–63, the export from India, and the destination of supplies, were as follows:—
| Destination. | Quantity. | Value. | |
| United Kingdom | 8,537,133 | lbs. | $1,627,035 |
| America | 134,064 | 26,949 | |
| Arabian and Persian Gulfs | 343,037 | 33,385 | |
| France | 1,922,120 | 371,396 | |
| Germany | 85,680 | 15,504 | |
| Suez | 295,269 | 51,730 | |
| Other places | 9,577 | 815 | |
| Total | 11,326,880 | lbs. | $2,126,814 |
The value of exports in 1866 was £1,861,501. In the same year the imports of indigo from the whole of Central America, including Honduras, was 672,480 lbs. The consumption of indigo in Great Britain did not increase during the ten years ending with 1867. This stationary demand, notwithstanding the fall in the price of the drug and increase of population, is attributed by McCulloch principally to the decreasing use of blue cloth. It is more probably due to the substitution of cheaper dyes. The average home consumption in Great Britain for seven years ending in 1867, was 1,675,072 lbs. per year.
The importation into this country for the twenty years last past is shown by the following table, kindly prepared at our request by the chief of the Bureau of Statistics:—
26
Statement of Imports of Indigo into the United States during the Fiscal Years ended June 30, 1853–1872.
| Fiscal Years ended June 30. |
INDIGO. | |||
| FREE OF DUTY. | DUTIABLE | |||
| Pounds. | Dollars. | Pounds. | Dollars. | |
| 1853 | 1,387,847 | 947,367 | ||
| 1854 | 1,965,789 | 1,282,367 | ||
| 1855 | 2,097,397 | 1,151,516 | ||
| 1856 | 1,732,290 | 1,063,743 | ||
| 1857 | 1,533,037 | 1,010,509 | ||
| 1858 | 1,647,767 | 945,083 | ||
| 1859 | 1,773,868 | 1,441,429 | ||
| 1860 | 1,707,116 | 1,413,790 | ||
| 1861 | 185,039 | 160,138 | 719,563 | 505,766 |
| 1862 | 2,501,052 | 3,281,441 | ||
| 1863 | 885,834 | 1,008,187 | 178,364 | 219,169 |
| 1864 | 684,813 | 623,406 | 897,821 | 671,899 |
| 1865 | 741,438 | 601,283 | 415,575 | 324,207 |
| 1866 | 798,855 | 609,160 | 44,660 | 41,268 |
| 1867 | 1,069,506 | 816,974 | ||
| 1868 | 870,164 | 775,751 | ||
| 1869 | 1,574,449 | 1,649,550 | ||
| 1870 | 1,270,579 | 1,203,664 | ||
| 1871 | 1,994,172 | 2,052,222 | ||
| 1872 | 1,526,869 | 1,484,744 | ||
| 1854 | ||||
EDWARD YOUNG, Chief of Bureau.
Bureau of Statistics, Nov. 16, 1872.
The extraordinary quantity imported in 1862, we hardly need remark, was due to the demand for consumption in army cloths. Indigo imported directly, was made free of duty in 1861. The duty which appears by the above table to have been charged since that period, was upon indigo, the product of India, imported by way of England, which was subject to an extra duty of ten per cent.
The indigo consumed in the United States is generally supplied by the Boston and New York Calcutta houses, who have either an American partner resident in Calcutta, or who employ a resident American as agent. Indigo, like other Calcutta goods, 27 is sold through the agency of brokers, who receive on this article a commission of one per cent. The value of the article is known almost daily in these cities by telegrams, giving exact information of the state of the trade, transmitted from Calcutta as often as every five days. Some of the brokers publish monthly circulars, showing the stock of indigo with other Calcutta goods on hand in our market. The regular trade reports issued by the India merchants show that The higher qualities of indigo do not come to our market. The following is an extract from a report of Whitney, Brother, & Co., of 1871:—
| Indigo for Continent | fine | 350 to 362 | rupees. |
| „ „ „ | good | 330 „ 345 | „ |
| „ „ „ | middling | 310 „ 325 | „ |
| American consuming | fine | 280 „ 300 | „ |
| „ „ | good | 250 „ 275 | „ |
| „ „ | middling | 200 „ 240 | „ |
| „ „ | low and ordinary | 150 „ 170 | „ |
At the present moment there is great depression in the trade in this article. The last telegrams show a decline of price in the Indian trade in this article of from fifty to seventy-five per cent from the prices of last year; and the apprehension is even entertained that indigo is going out of use, the dreaded competitors being the aniline dyes, and particularly the Nicholson blue. We maybe presumptuous in giving our opinion on the question, but we hazard the prediction that, notwithstanding the temporary popularity of the cheap substitutes, a reaction will take place in favor of that “wonderful and most valuable production,” whose importance as a dye has been held in India for thousands of years and Europe for two centuries, “greatly to exceed any other.” [2]
The “Dictionnaire Universel du Commerce,” &c., published in 1861, contains an exhaustive article on the commerce in indigo, by M. S. Beekrode. From the statements of this writer, it appears that the consumption of indigo was estimated, in 1835, as follows:—
| Great Britain | 1,214,380 | kilograms | (2,683,779) | lbs. |
| France | 912,915 | „ | (2,017,542) | „ |
| United States | 130,000 | „ | (277,300) | „ |
| Other countries | 2,435,473 | „ | (5,382,395) | „ |
| Total | 4,692,768 | kilograms | (10,362,016) | lbs. |
The approximate consumption in 1859 is stated as follows:—
| Great Britain | 800,000 | kilograms | (1,768,000) | lbs. |
| France | 800,000 | „ | (1,768,000) | „ |
| United States | 400,000 | „ | (884,000) | „ |
| Russia | 860,000 | „ | (1,900,600) | „ |
| The Zollverein | 1,250,000 | „ | (2,762,500) | „ |
| Switzerland | 150,000 | „ | (331,500) | „ |
| Austria | 400,000 | „ | (884,000) | „ |
| Other countries | 300,000 | „ | (663,000) | „ |
| Total | 4,960,000 | kilograms | (10,961,600) | lbs. |
The average production in 1859 is estimated as follows:—
| Bengal, Madras, &c. | 3,500,000 | kilograms | (7,735,000) | lbs. |
| Java | 550,000 | „ | (1,215,500) | „ |
| Central America | 300,000 | „ | (884,000) | „ |
| Other sources | 100,000 | „ | (221,000) | „ |
| Total | 4,450,000 | kilograms | (9,834,500) | lbs. |
As the maximum annual consumption in 1859 is set down at 5,000,000 kilograms, the author concludes that the average production at that time did not surpass the requirements of the dyers of the whole world.
28
As pertinent to the commercial branch of our subject, we must briefly notice the remarkable facts of the sudden growth and equally sudden and extraordinary extinction of the production of indigo in the Carolinas. Indigo was for many years the second great staple of South Carolina. So highly was this staple estimated that the historian of the State declares that “it proved more really beneficial to Carolina than the mines of Mexico or Peru are or ever have been to Old or New Spain.” Its introduction was the happy result of a woman’s culture and energy. In the early part of the last century, the indigo plant had been extensively cultivated in the West India Islands, which then furnished the chief supply of Europe. The governor of Antigua, George Lucas, whose home plantation was at Wappoo in Carolina, having observed the fondness of his daughter, Miss Eliza Lucas, afterwards the mother of General Charles Cotesworth Pinckney, for the culture of plants, was in the habit of sending to her tropical seeds to be sowed on his plantation at Wappoo. Among others, he sent her some seeds of the indigo plant cultivated in the West Indies. She planted them for two years; but 29 the seeds failed to germinate, or were killed by the frost. On the third year’s trial, in 1741 or 1742, she was successful. Governor Lucas, on hearing that the plants had ripened and produced seed, sent from Montserrat a person skilled in making indigo. Vats were built on Wappoo Creek, and there the first American indigo was manufactured. The attempts of the expert to conceal his processes were defeated by the vigilance of Miss Lucas. The process of manufacture was made known. Seeds from the Wappoo plantation were freely distributed and successfully planted; and the culture of indigo became common. In 1747, a considerable quantity of indigo was sent to England, which induced the merchants trading with Carolina to petition parliament for a bounty on Carolina indigo. In 1748, an act of parliament was passed granting a bounty of sixpence per pound on indigo raised on British-American plantations and imported directly to Britain from its place of growth. This act stimulated the planters of Carolina to double vigor in the production of this new material for export. “Many of them,” says Dr. Ramsay, “doubled their capital every three or four years by planting indigo.” In the year 1754, the export of indigo from the province amounted to 216,924 lbs., and in the years 1772 and 1773 the export had risen to 1,107,661 lbs. The production was greatly checked by the war of the Revolution. Near the close of the century the large importations from India lowered the price, so as to make the planting unprofitable. In the mean time, the culture of cotton had sprung up under the protective tariff of 1789. The grounds suitable for indigo planting were equally fitted for cotton, and were for the most planted with the new staple. It is curious to observe how the former was displaced by the latter staple. The export of indigo from Charleston in 1797 was 96,121 lbs.: in 1800, it fell to 3,400 lbs. During the same years, the exports of cotton rose from one million to six and a half million pounds. The production of American indigo appears to have revived from time to time up to 1829. A writer of that period in Silliman’s Journal of Science estimates—although it would seem on doubtful authority—the production of indigo in the United States at 20,000 lbs. The price 30 of the American article had fallen, owing to the great quantity of extractive which it contained, to fifty cents per pound, while the Bengal indigo was worth $1.15 per pound. We have no data as to its production at the present time, but infer, from the fact that no reference has been made to this product in the Government Agricultural Reports for many years past, that the production, if any, is too unimportant to be noticed.
All the applications of indigo require that the material should first be reduced to an impalpable powder. It is better to grind it with water, to prevent the loss of material in the form of powder, although the dry pulverization is necessary when the indigo is to be used for the manufacture of the sulphate. To facilitate the grinding the material into a paste, it should be previously soaked in hot water from one to three hours. The grinding on a small scale may be done by a very simple apparatus. This is a hemispherical vessel of copper or cast-iron, eighteen inches in diameter, furnished at the edge with two handles. The workman, sitting astride a bench, places the vessel before him, in which he places three heavy cast-iron balls, the indigo which has been softened, and a sufficient quantity of water. Holding the basin by the handles, he gives it a circular oscillatory movement, in such a manner that the balls, following this movement, crush the indigo which surrounds them; after which the contents are poured into another vessel, water is added, and the material is stirred. The portions incompletely ground are made to reunite themselves at the bottom by means of regular blows with a hammer on the rim of the vessel. The upper liquid is decanted, and the deposit is submitted to a new manipulation in the basin.
In large establishments the grinding is done by machinery. An apparatus highly recommended, consists of two circular plates of cast-iron, arranged horizontally and slightly separated, one from the other, which are rapidly rotated by power, in inverse, directions. The interior surfaces of these disks are provided with deep grooves radiating in a curved line from the 31 centre to the circumference, and diminishing in depth in the same direction. The indigo which has been previously softened enters between the two plates by an opening in the centre of the upper one, and escapes in a thin paste by the circumference.
The application of indigo to the coloring of textile fabrics requires the complete dissolving of the substance, for which the mechanical division is only a preliminary. There are only two known means of dissolving this substance: 1. By reduction; 2. By the action of concentrated sulphuric acid. The first means allows indigotine to be regenerated; and, when the dyeing is completed, it is pure indigotine which adheres to the colored fibre. By the second means, or dissolving by sulphuric acid, the coloring material enters into a new combination, from which it can never be separated: it becomes a new substance, endowed with new and special properties.
The fixing of Indigotine by means of Reduction.—In this method the operator avails himself of one of the most remarkable qualities of indigotine: this is the facility with which this body takes up hydrogen, and becomes transformed into a colorless substance, which is soluble in favor of alkaline or alkaline-earthy bases, and is susceptible of reproducing indigotine by simple oxidation in contact with air. This hydrogenized substance is called white indigo. Blue indigo, or indigotine, is insoluble except by concentrated sulphuric acid; and this insolubility gives it its superiority to all other blue dyes. Not being soluble, it cannot, as blue indigo, attach itself to the material to be dyed; but in the soluble form of white indigo it can perfectly penetrate the fibre. If by any means of oxidation we can transform the white indigo into blue indigotine, the latter becomes insoluble, and is imprisoned in the pores of the fibre. This is, briefly, the whole theory of the use of indigo in dyeing or printing, although the reaction may be applied in different ways to the coloring of fibres, such as—
1. The indigo is dissolved by means of an alkaline reduction in a vat, and the fibre is immersed in the bath. This is the common blue vat.
2. The solution prepared beforehand is painted by a hair 32 pencil and printed by a stamp or roller upon only certain parts of the tissues. This is the pencil blue.
3. The white indigo is precipitated under the form of a paste, in combination with a metallic oxide having strong reducing power, such as hydrated protoxide of tin, which prevents the too rapid reoxidation of the indigotine. The thickened paste is printed, and the tissue is placed in an alkaline bath (lime or soda), which, displacing the oxide of tin, forms a soluble combination of white indigo. The latter can then penetrate the fibre, and afterwards become fixed by reoxidation. This is the printer’s solid blue.
4. The finely ground, but not dissolved indigo, is placed upon the tissue in such conditions that it can be dissolved and reduced in place. This done, the fixing of the indigotine is effected by oxidation. This is the method for China blue or bleu faïence.
Without dwelling upon the details of these methods, we hasten to a consideration of the most important of all the applications of indigo:—
The Copperas Vat.—For dyeing cotton, the method of reduction found by experience to be the most convenient and practical is founded upon the action of the hydrate of the protoxide of iron in the presence of lime. The hydrated protoxide of iron is obtained from sulphate of iron (green vitriol, or copperas) with freshly burned lime. Certain precautions should be observed in the use of these materials. The copperas used for the preparation of these vats should be free from sulphate of copper, because the oxide of copper which would be formed in the vats rapidly oxidizes the reduced indigo, and causes its precipitation in the bath. The copperas ought not to contain red oxide of iron, nor sulphate of alumina. The coppery or oxidized vitriol may be purified by boiling the solution with pieces of iron, which precipitates the iron and neutralizes the oxide. The lime ought to be pure, containing no magnesia; when slacked lime has been exposed to the air, even for a short time, it absorbs carbonic acid, and becomes converted into chalk. The lime, therefore, 33 should always be newly slacked. The ingredients, then, of a copperas vat are water, pure or purified green vitriol, indigo ground into a homogeneous impalpable paste, and pure and freshly slacked lime. The proportions used in different establishments are exceedingly variable. Those which answer for a laboratory vat, or a small vat used for precipitating the white indigo immediately for printing, are: indigo, one part; sulphate of iron, two parts; slacked lime, three parts. These proportions are not enough for the large vats used in dyeing pieces. In them it is necessary to make the quantities of lime and sulphate of iron larger than the theory of the vat requires. The excess of lime and hydrate of iron serve the purpose, whenever the vat is stirred, to repair the losses of indigo caused by its oxidation from contact with the air. Schutzenberger gives the proportions generally used by the dyers of France, as follows:—
| Indigo | 1 | part. |
| Crystallized sulphate of iron | 3 | „ |
| Freshly slacked lime | 3 | „ |
Others, he says, use more lime than copperas, as in the following proportion:—
| Indigo | 2 | parts. |
| Sulphate of iron | 5.5 | „ |
| Quicklime | 6.5 | „ |
M. de Kæppelin, who is especially familiar with the cotton dyeing in Mulhouse, describes the ordinary vats for cotton dyeing as bound with iron, and placed on the level of the ground. They hold from 3,000 to 4,000 litres (1,055 gallons) of liquid. In preparing them the dyer fills them about three-quarters full of water, and pours in a milk of lime, prepared with 45 kilograms (100 lbs.) of freshly slacked lime; a fine liquid paste having been previously made from 15 kilograms (33 lbs.) ground in water. This is added to the lime in the vat by portions, the liquid in the vat being stirred up by a rake after each portion of the indigo paste has been added. The indigo becomes dissolved in about twenty-four hours, when the vat can be used. After describing the manner in which the frame, or champignon, containing the goods to be 34 dyed is arranged and immersed in the vat, this author continues: “It will be understood that the vat is composed according to the degree of intensity of the color which is sought to be obtained, and that hues more or less deep may be obtained by means of more or fewer repeated immersions of the fabric to be dyed. After each immersion the champignon is lifted out of the vat, and the fabrics are left to ungreen themselves by contact with the air. (It must be observed that, although soluble indigo is called white, because it is without color when carefully prepared in the laboratory, the goods, when first taken from the ordinary vat, are of a green color.) Exposed to the air, the soluble indigo is precipitated in the state of blue indigo upon the fibres of the tissue. This oxidation, or dehydryzation, may be hastened by plunging the tissue into a vat containing a solution, very much diluted with water, chloride of lime, bichromate of potash, or sulphuric acid. The first two act as oxidizing agents; the last facilitates the restoring of the blue indigo by depriving it of the lime which is in excess in the solution of indigo which the tissue has imbibed from the vat.”
He adds further: “To facilitate the formation of blue indigo in the interior of the fabrics, the stuff to be dyed may be previously impregnated by a saline solution, which has the property of precipitating the white indigo from the alkaline solution, and of fixing itself more rapidly upon the tissue. Oxide of copper and oxide of manganese possess these properties in a high degree, and are used in many establishments to hasten the dyeing process, and produce an economy in raw materials. The pieces of cloth are placed in a solution of sulphate of copper, in the proportion of 15 to 20 grams to the litre (2.11 pints), and lightly thickened with starch. The fabrics, thus impregnated by a kind of mordant, before receiving the blue dye are first passed through a weak bath of milk of lime, which fixes the oxide of copper upon the tissue. The blues thus obtained are more intense, and have a peculiar lustre. This process is used in Austria and Germany, where cotton fabrics are printed on both sides of the tissue.”
Coming to the English authorities, Dr. Grace Calvert, in 35 his recent lectures before the Society of Arts, speaking of the cold vat for dyeing cotton, says: “The oldest, and still most generally employed method of preparing cold vats, consists of putting into a vat containing about 2000 gallons of water 60 lbs. of indigo, very finely powdered, 180 lbs. of slacked lime, and 120 lbs. of sulphate of protoxide of iron, or green vitriol (free from any trace of copper salt), the two latter substances being added from time to time. The greater part of the lime used unites with the sulphuric acid of the iron salt, to produce sulphate of lime or gypsum; and the liberated protoxide of iron removes the oxygen from the indigo, becoming converted into saline oxide, whilst the reduced indigo dissolves in the excess of lime employed.”
He adds the following facts, which may be of practical value:—
“Messrs. R. Schloesser & Co., of Manchester, have introduced within the last year or two a marked improvement, in the preparation of cold vats, which removes the great objections of the bulky precipitate of sulphate of lime, the formation of an oxide of iron, and the loss of indigo by its combination with the oxide of iron. The bath remaining much more fluid, the pieces are less apt to be spotted, and a better class of work is produced. To carry out their process, they add to the ordinary 2,000 gallon vat 20 lbs. of ground indigo, 30 lbs. of iron borings, 30 lbs. of their remarkable powdered zinc, and 35 lbs. of quicklime; the whole is stirred up from time to time, for twenty-four hours, when it is ready for use. If the bath is not considered sufficiently strong, a little more lime and zinc are introduced. The chemical theory of the process is, that the zinc, under the influence of the lime, decomposes the water, combining with its oxygen, and the hydrogen thus liberated removes oxygen from the indigo which then dissolves in the lime.”
An excellent description of the processes employed at Manchester, England, in preparing and working the copperas, or cold vat, is given in Ure’s “Dictionary of Manufactures.” “The ingredients necessary for setting the vat are copperas, newly slacked quicklime, and water. Various proportions of 36 these ingredients are employed, as, for instance: 1 part by weight of indigo (dry), 3 parts of copperas, and 4 of lime; or, 1 of indigo, 2¹⁄₂₃ of copperas, and 3 of lime; or, 8 of indigo, 14 of copperas, and 20 of lime; or, 1 of indigo, ¾ of copperas, and 20 of lime; or 1 of indigo, 4 of copperas, and 1 of lime. The sulphate of iron should be as free as possible, from red oxide of iron, as well as sulphate of copper, which reoxidize the reduced indigo-blue. The vat, having been filled with water to near the top, the materials are introduced, and the whole, after being well stirred several times, is left to stand for about twelve hours. The chemical action which takes place is very simple. The protoxide of iron, which is set at liberty by the lime, reduces the indigo-blue; and the indigo, which is then dissolved by the excess of lime, forming a solution, which, on being examined in a glass, appears perfectly transparent and of a pure yellow color, and becomes covered, whenever it comes in contact with the air, with a copper-colored pellicle of regenerated indigo-blue. The sediment at the bottom of the vat consists of sulphate of lime, peroxide of iron, and the insoluble impurities of the indigo, such as indigo-brown in combination with lime, as well as sand, clay, &c. If an excess of lime is present, a little reduced indigo-blue will also be found in the sediment in combination with lime. The dyeing process itself is very simple. The vat having been allowed to settle, the goods are plunged into the clear liquor, and, after being moved about in it for some time, are taken out, allowed to drain, and exposed to the action of the atmosphere. While in the liquid, the fabric attracts a portion of the reduced indigo-blue. On now removing it from the liquid, it appears green, but soon becomes blue on exposure to the air, in consequence of the oxidation of the reduced indigo-blue. On again plunging it into the vat, the deoxidizing action of the vat does not again remove the indigo-blue which has been deposited within and around the vegetable or animal fibre, but, on the contrary, a fresh portion of the reduced indigo-blue is attracted, which, on removal from the liquid, is again oxidized like the first, and the color thus becomes a shade darker. By repeating this process several 37 times the requisite depth of color is attained. This effect cannot, in any case, be produced by one immersion in the vat, however strong it may be. The beauty of the color is increased by finally passing the goods through diluted sulphuric or muriatic acid, which removes the adhering lime and oxide of iron. After being used for some time, the vat should be refreshed or fed with copperas and lime, upon which occasion the sediment must first be stirred up, and then allowed to settle again, so as to leave the liquor clear. The indigo-blue, however, is in course of time gradually removed, and by degrees the vat becomes capable of dyeing only pale shades of blue. When the color produced by it is only very faint, it is no longer worth while using it, and the contents are then thrown away. In dyeing cotton with indigo, it seems to be essential that the reduced indigo-blue should be in contact with lime. If potash or soda are used in its place, it is impossible to obtain dark shades of blue.”
The application of indigo-blue to wool and woollen tissues is always made by means of vats, which have special names; as, the pastel or woad vat, the urine vat, German vat, molasses vat, &c. The reduction or hydrogenation of the indigo-blue is the result of a peculiar fermentation, which is developed within an alkaline liquor by means of nitrogenized substances and bodies rich in sugar or hydrocarbonized substances. It is known that in these conditions, especially where the temperature is slightly raised, the sugar is converted into butyric acid, and at the same time carbonic acid and hydrogen are set free. We find here the source of the nascent hydrogen which fixes itself upon the indigo-blue, and transforms it into white indigo, which is soluble in the alkalies of the vat. It has recently been observed that the butyric fermentation proceeds from the development of minute infusoria. These animalculæ live without any supply of oxygen, and, in fact, are killed in its presence. They therefore live at their ease in the vat of reduced indigo, where no oxygen is permitted to enter.
The ingredients most usually employed for furnishing the 38 hydrocarbonaceous substances for fermentation are bran and ground madder, although molasses is sometimes used. The nitrogenized material is found in the woad or pastel, which is often added in very large proportions to the fermenting vats. It is observed by the chemists who have studied this subject most carefully, that the preparation of vats, founded upon the principle of fermentation, does not repose upon principles so sure and constant as those of the copperas vat, and that many unforeseen accidents interpose to disturb the work of an inexperienced dyer. The phenomena in fermentations are often complex. It is admitted that in these phenomena theory has not said its last word, and that empiricism is often more fortunate than science. In conducting the operations of the warm fermenting vat, the conceit of the practical dyer, so often remarked upon, is not without foundation. By practical experience and the traditions of his art he has acquired a knowledge of the almost insensible modification in conditions which can change or arrest the chemical reaction. It is the knowledge of the workman, a knowledge almost instinctive, which can never be communicated to the books, and which is most respected by those most profoundly informed in theory.
The Woad or Pastel Vat.—In former times woad, already referred to, was the only material known to the dyers of Europe for producing the blue color of indigo. For dyeing wool, the use of woad, now abandoned wholly in cotton dyeing, has been retained to the present day, generally for the purpose of exciting fermentation, and without regard to its effect in imparting color to the material to be dyed; for the woad grown in England, and used in the dye-houses of that country, contains no trace of coloring matter. The woad, or pastel, grown in the warmer districts of France contains about two per cent of indigotine, which is regarded in that country as an important addition to the coloring material, especially for improving the tone of the color. Various substitutes, such as rhubarb leaves, turnip and carrot tops, and weld, have been tried, but without advantage, with the exception, perhaps, of weld, which is still used by some dyers. Some chemists regard the use of woad as 39 the remnant of a prejudice; but the better opinion is, that this material possesses peculiar fermentiscible qualities, whose exact action science has yet to resolve.
According to Schutzenberger, the most recent and highest French authority, the dimensions of the pastel vat are about 6½ feet in diameter, by 9 in depth. 100 kilograms (221 lbs.) of pastel, in balls, is placed in the vat, which is then filled with boiling water. To this is added 10 kilograms (22 lbs.) of madder, 3 to 4 kilograms (about 6½ to 8¾ lbs.) of bran, and 4 kilograms of quicklime, which has been slacked, and in the form of a bouilli. Sometimes weld is also added. After three hours of rest, the vat is well raked, and the operation is repeated every three hours. There is gradually developed a characteristic ammoniacal vapor, and a blue scum, with veins of deeper blue, forms on the surface; and the liquid, when agitated in the air, rapidly becomes blue. These symptoms indicate the dissolution of the indigotine of the woad; then there is added 10 kilograms (22 lbs.) of indigo which has been previously ground in water, and the vat is stirred. If the fermentation appears to be proceeding too actively, which is recognized by the disengagement of gases, it is checked by the addition of a proper dose of lime. On the other hand, the fermentation is made more active by increasing the dose of bran. The first dyes are not so good as those subsequently obtained, as the woad absorbs from the bath certain brown or yellow materials, kept in solution, and furnished as well by the pastel and madder as by the indigo itself. 100 kilograms of wool require from 8 to 12 kilograms of indigo. The vat is kept up by successive additions of indigo and lime, made in the evening.
Another kind of pastel vat, prepared much like the last, receives an addition of a dose of potash. M. de Kæppelin describes it as the one at present in general use in France. Into a vat containing from 3,000 to 4,000 litres (791 to 1,055 gallons) there is placed 75 kilograms (166 lbs.) of pastel in loaves, or which has undergone a kind of fermentation; or, what is preferable, 80 to 100 kilograms (176 to 221 lbs.) of pastel or woad gathered without fermentation, and 10 kilograms (22 lbs.) of 40 indigo, ground to a paste with water. This mixture is well stirred, and there is added 4 kilograms (about 9 lbs.) of Avignon madder, and the same quantity of carbonate of potash. After the vat has been well raked there is added 2 kilograms (4½ lbs.) of slacked lime, and some pails of bran. The vat is well covered, either with a wooden lid, or woollen cloths. The fermentation is allowed to proceed, and after five or six hours the vat is uncovered and raked with much care for half an hour. This operation is repeated every three hours, until it is recognized that the indigo is well dissolved. In this case the bath ought to be of a beautiful yellow color, and be covered with a light blue irised film, veined with yellow at the least movement given to the liquid. If the fermentation proceeds too rapidly, a little lime is added to moderate it.
For keeping up a vat like this, and to obviate the different inconveniences to which it is subject, the dyer sometimes adds lime, or sugar, and carbonate of ammonia, sometimes madder, or bran, or even tartar-lees. These last additions are made to saturate the excess of lime which the vat contains. In this case the yellow veins and the beautiful blue scum which cover the surface disappear, or become pale; a piquant odor is disengaged, and the liquor becomes blackish. When lime or sugar are added, it is for the purpose of retarding the fermentation of the woad. Sugar might even entirely take the place of pastel for effecting the reduction of the indigo, and many establishments in France are commencing to use it for this purpose. A good vat, well supplied with successive additions of indigo, pastel, bran, and madder, in proportions necessary to effect and prolong the fermentation necessary for the dissolution of the indigo, may be kept up many years.
Schutzenberger observes that the vats of fermentation are subject to certain maladies, the two most frequent of which are due, one to an excess, and the other to an insufficient quantity of lime. “In the first case, the liquid takes a tint more and more free of color, loses its fleurée (surface scum) and odor; the fermentation is then arrested by the precipitation of the active matters. This inconvenience is remedied, if seen in time, by 41 adding sulphate of iron, which eliminates the too great excess of lime. In the second, the fermentation becomes too active, passes into a putrid fermentation, and the liquid assumes a reddish tint; a fabric dyed with indigo in this state becomes very soon discolored. The sole means of safety is to heat the bath up to 90° and to add lime. If this does not accomplish the purpose of arresting the putrefaction the vat is lost.”
The following account of the method of dyeing woollen goods with indigo by means of the woad vat is given by Dr. Ure, as that carried on in Yorkshire, the great centre of the woollen manufacture of England.
“The dye-vats employed are circular, having a diameter of six feet six inches, and depth of seven feet, and are made of cast-iron five-eighths of an inch in thickness. They are surrounded by brickwork, a space of three inches in width being left between the brickwork and the iron, for the purpose of admitting steam, by means of which the vats are heated. The interior surface of the brickwork is well cemented. In setting a vat the following materials are used: 5 cwt. of woad, 30 lbs. of indigo, 56 lbs. of bran, 7 lbs. of madder, 10 quarts of lime. The woad supplied to the Yorkshire dyers is grown and prepared in Lincolnshire. It is in the form of a thick, brownish yellow paste, having a strong ammoniacal smell. The indigo is ground with water in the usual manner. The madder acts in promoting fermentation, but it also serves to give a reddish tinge to the color. The lime is prepared by putting quicklime into a basket, then dipping it in water for an instant, lifting it out again, and then passing it through a sieve, by which means it is reduced to a fine powder, called by the dyers ware. The vat is first filled with water, which is heated to 140° F., after which the materials are put in, and the whole is well stirred until the woad is dissolved or diffused, and it is then left to stand undisturbed overnight; at six o’clock the next morning the liquor is again stirred up, and five quarts more of lime are added; at ten o’clock five pints of lime are again thrown in, and at twelve o’clock the heat is raised to 120° F., which temperature must be kept up until three o’clock, when another quart of lime 42 is introduced. The vat is now ready for dyeing. When the process of fermentation is proceeding in a regular manner, the liquid, though muddy from insoluble vegetable matter in suspension, is of a yellow or olive yellow color; its surface is covered with a blue froth or copper-colored pellicle, and it exhales a peculiar ammoniacal odor; at the bottom of the vat there is a mass of undissolved, matter of a dirty yellow color. If there is an excess of lime present, the liquor has a dark green color, and is covered with a grayish film, and, when agitated, the bubbles which are formed agglomerate on the surface, and are not easily broken. Cloth dyed in a liquid of this kind loses its color on being washed. This state of the vat is remedied by the addition of bran, and is of no serious consequence. When, on the other hand, there is a deficiency of lime, or, in other words, when the fermentation is too active, the liquor acquires first a drab, then a clay-like color; when agitated, the bubbles which form on its surface burst easily, and when stirred up from the bottom with a rake it effervesces slightly, or frits, as the dyers say. If the fermentation be not checked at this stage, putrefaction soon sets in, the liquid begins to exhale a fetid odor, and when stirred evolves large quantities of gas, which burns with a blue flame on the application of a light. The indigo is now totally destroyed, and the contents of the vat may be thrown away. No further addition of woad is required after the introduction of the quantity taken in first setting the vat, the fermentation being kept up by adding daily about four pounds of bran with one quart or three quarts of lime. Indigo is also added daily for about three or four months. The vat is then used for the purpose of dyeing light shades, until the indigo contained in it is quite exhausted, and its contents are then thrown away.”
This author adds: “Woollen cloth, before being dyed, is boiled in water for one hour, then passed immediately under cold water. If it be suffered to lie in heaps after being boiled it undergoes some change, which renders it afterwards incapable of taking up color in the vat. In dyeing, the cloth is placed on a net-work of rope attached to an iron ring, which is suspended by four iron chains to a depth of about three feet beneath the surface of the 43 liquor. The cloth is stirred about in the liquor by means of hooks for about twenty or thirty minutes. It is then taken out and well wrung. It now appears green, but, on being unfolded and exposed to the air, rapidly becomes blue. When the vat has an excess of lime the cloth has a dark green color when taken out. It is then passed through hot water, and dipped again if a darker shade is required.”
The Indian Vat.—This presents much analogy to the woad vat, as the fermentation of vegetable matters effects the transformation of the indigo-blue. According to Dr. Calvert, the Indian vat, probably so called from its origin in the East, is taking the place in England of the old woad vat for dyeing wool and woollens. He describes its preparation as follows: 8 lbs. of powdered indigo is added to a bath containing 3½ lbs. of bran, 3½ lbs. of madder, and 12 lbs. of potash, which is maintained for several hours at a temperature of 200° F. It is then allowed to cool to 100° F., when fermentation ensues. After about forty-eight hours the indigo is rendered soluble, being reduced by the decomposition of the sugar and other products contained in the bran and the madder root during the process of fermentation. The distinguishing feature of this vat is the use of potash. The Indian or potash vats are spoken of by the best authorities as more easy to manage than the woad vat. They are less subject to accidents, and yield their coloring material more readily to the fibre, while three times as much wool can be dyed in the same time. On the other hand, they do not last so long, and require to be renewed at the end of twenty-five or thirty days. Besides, the fibres dyed in the potash vat have a darker shade than those dyed in the woad vat, owing to the large quantity of the coloring matter of the madder dissolved by the potash, which becomes fixed on the stuff with the indigo-blue.
The Urine Vat, but little used except for domestic dyeing, is founded upon the same principles as the other fermenting vats. This excretion, when putrefied, contains at the same time the nitrogenized principles which work as ferments and the alkali in the form of ammonia necessary for dissolving the indigo.
According to Dr. Calvert, improvements have been made of 44 late years in the fermenting indigo vats by which the expense of madder is avoided. They are now prepared by adding to water, at a temperature of 200° F., 2 buckets of bran, 26 lbs. of soda crystals, 12 lbs. of indigo, and 5 lbs. of slacked lime. After five hours the bath is allowed to cool to 100° F., when fermentation ensues, and the indigo is dissolved in the alkali. This is, in fact, the German vat, soda taking the place of the potash, and the only fermenting material consisting of bran.
The German Vat is largely used by the dyers in the north of France, and is considered as more advantageous than the Indian vat, because the employment of soda is more economical than that of potash, while the vat can be maintained as long as two years. The vats used by them are prepared as follows: The water is heated to a temperature of 95°, and receives 20 pails of bran, 11 kilograms (about 24 lbs.) of crystals of carbonate of soda, 5.5 kilograms (11 lbs.) of indigo, and 4½ lbs. of slacked lime. After twelve hours, the temperature having been kept at 40° or 50°, fermentation commences, the liquid becomes of a greenish blue color, and disengages bubbles of gas. Indigo, soda, and lime are put in from time to time in the proportions above indicated, and also from six to eight pounds of molasses. At the end of the third day the vat is fit for use.
M. de Kæppelin, writing in. 1864, informs us that the reduction of indigo by means of molasses, is at present largely employed in the great establishments for dyeing woollen cloth at Sedan, Louviers, and Elbœuf.
The vat used is of very large dimensions, and from twenty-two to twenty-six pounds of indigo are dissolved in it; an equal weight of molasses is used, and three or four times the same weight of potash made caustic by a proportionate addition of lime.
The space reserved for this subject in our present paper will not permit us to enter upon a description of the processes used in the American dye-houses. This, as well as the applications of indigo in printing, and the uses of sulphate of indigo, must be deferred to another number.
Let us, in concluding the first part of our paper, at the risk of 45 repetition, bring out in bolder relief a statement which presents the philosophy of all the various processes of the indigo vat, and at the same time, a conclusive argument for the use of this material, in preference to all cheaper substitutes. Indigo cannot enter into a fibre until it is dissolved. It cannot be dissolved so long as it is in a blue state. When reduced by any of the processes above described to the white state, it is easily dissolved, and can enter the pores of the fibre. Upon exposure to the oxygen of the air it takes up an equivalent of oxygen; it returns to the blue state, and, being then insoluble, it cannot be washed away from the fabric, and being saturated with oxygen it cannot be changed by air or light. This theory of the application of indigo involves a lesson to manufacturers, dealers, and consumers, especially of woollen fabrics. The theory, as well as experience, dating back to the dawn of the textile arts in the East, establishes that this material is incalculably superior to any other, in permanence at least, for imparting to woollen fibre a blue color, or as a foundation for most of the darker colors. By far the largest proportion of all cloths are of dark colors,—blue, black, green, brown, gray, or mixed,—and can advantageously receive in all or a portion of the fibre constituting them a direct dye or bottom for other dyes from indigo. It may be safely stated that, as a whole, no cloths in the world are manufactured from such good wool as those produced in the United States. We might expect that the shoddy goods of Yorkshire should be further falsified by fugacious dyes; but is it not a shame that our admirable wool should be deprived of half its value by parsimony in dyeing? The slightest shortcomings in dyeing are revealed in wear. The writer cannot forbear referring to an illustration directly before his eyes. He is wearing a garment, reduced now to the retired service of an office coat, made of an admirable cheviot cloth of American manufacture. The cloth originally was selected not only for its excellent texture, but as an illustration of philosophical principles applied in the formation of color. The tissue was made by weaving three yarns of distinct colors,—blue, yellow, and red. Either of those hues alone would have been 46 glaring and conspicuous, but, by the law of color, the combination of blue, red, and yellow makes black, and the new cloth at a distance had the effect of a dark mixture. Upon exposure to ordinary wear, the yellow and red have retained their pristine hues; the blue, not being indigo dyed, has faded; and the original dark mixture, although sound in fabric, has become of a yellowish brown. The extra expense of a permanent dyeing material forms so small a proportion of the whole cost of a finished garment, that it ought not to be generally spared. The reform cannot be made by the manufacturers; it must be made by the dealers, and especially by that class of producers which has risen in our day into such great importance,—the manufacturers of ready-made clothing. If they would demand of the manufacturers, and furnish to their customers cloths more permanently dyed, it would be another step in the direction to which these establishments are tending,—the supply of the chief portion of the woollen clothing of the people. The manufacturers would gladly aid them; for it is the growing sentiment of American manufacturers that all their productions should be, in the proverbial phrase adopted from the dye-house, as expressing the highest excellence,—true blue.
47
Citations of authorities having been but partially made in the preceding article, the writer, for the purpose of giving his sources of information, and for the convenience of those who wish to pursue the subject further, appends a list of the more important works which he has consulted:—
Schutzenberger’s Traité des Matières Colorantes, t. ii. (the most recent and best modern authority); Bancroft’s Philosophy of Permanent Colors, vol. i.; Edinburgh Encyclopædia; Berzelius, Traité de Chimie, t. vi; Chevrueil, Leçons de Chimie Appliquée à Teinture, t. iii.; Dumas, Chimie Appliquée aux Arts, t. viii; Wurtz, Dictionnaire de Chimie, 1872, art. Indigo; Indigo et son Emploi, par De Kæppelin; Annales du Génie Civil, 1864, t. iii.; Lectures of Dr. Grace Calvert, Chemical News, Aug. 9 and 23, 1872; O’Neill’s Dictionary of Dyeing and Printing; Napier’s Chemistry Adapted to Dyeing; Muspratt’s Chemistry Applied to the Arts, articles Indigo and Dyeing; Ure’s Dictionary of Manufactures, ed. of 1860; Proceedings of Royal Society, vol. xvi.; Proceedings of Literary and Philosophic Society of Manchester, vol. iv.; McCulloch’s Dictionary of Commerce, ed. 1869; Dictionnaire Universel du Commerce, &c., ed. 1861; South Carolina Production.—Ramsay’s History; Drayton’s South Carolina; Silliman’s Journal, vol. xviii. A more complete bibliography is given in Schutzenberger’s work.
49
PART II.
51
We entered upon the subject of indigo, which we have treated at some length in our last issue, as much in the interest of the people as of manufacturers, for we were deeply impressed with the conviction that no improvement in our manufacturing processes would confer more benefit upon the masses than imparting stability of color to the clothing of the people. When one has a deep conviction upon a subject, upon which others have equal opportunities for judging, he may be sure that he is not alone in his impressions. He is moved by one of those waves of thought which, operating simultaneously upon many minds, gives that uniformity to public opinion at which we so often wonder. We are gratified to find, from responses to our last article, that we are not alone in our conviction of the importance of reviving “true blue” dyes. The head of a mercantile house, the extent of whose clientèle in mills both of wool and cotton is hardly surpassed, has assured us that we have not overstated the reform in dyeing which we have advocated. He had long shared in our convictions. Pointing to the throng of men in the crowded street, where we were conversing, he remarked that there was hardly a man in the crowd whose clothing would not have been improved by indigo dye. “The failure to use indigo dyes,” he 52 emphatically said, “costs the laboring people of this country millions of dollars every year. The fault is not to be charged to our own manufacturers alone; for the blue coat which I wear, and which I bought in Paris, annoys me by the crocking caused by its aniline dye.” In one very large mill of which he is director as well as selling agent, he is putting his principles in practice. All the heavy blue cloths intended for popular consumption are faithfully dyed, and each bears a stamp, “Warranted indigo dyed.” The ready-made clothing establishments which largely consume these goods have already found their advantage in purchasing them, and a similar stamp is attached to each article made from this cloth.
Some of our most celebrated cotton fabrics have won and still retain their reputation by the use of indigo dyes. The ginghams are a signal illustration. The blue check is formed by weaving cotton yarns dyed blue in the cold indigo vat with undyed yarns. These goods can be washed indefinitely without change.
Another illustration is the famous A.B.A. Amoskeag tickings, an article of such excellence that the question of the right to use trade-mark A.B.A. gave rise to the leading American case in this branch of law. [3] A prominent feature in these goods was and still is the permanence of the dye in the blue stripe, produced by the cold indigo vat. Still another illustration is the blue and white “shirting stripe” first made by Mr. Samuel Batchelder, at the Hamilton Mills, now so generally adopted for sailors’ shirts. The indigo dye enables the color to resist the roughest possible usage.
To recur to the application of indigo dyeing to wool and woollens. We have been unable, although we have written more than fifty letters of inquiry upon the subject, to learn of any peculiarity or improvements in the American processes of wool dyeing with indigo. [4] Our dyers are for the most part 53 foreigners. For this reason, or because the art of indigo dyeing has long since reached perfection in the best establishments abroad, they rigidly pursue the old European methods. The best dyers regard the successful management of the warm fermenting vats for wool as the highest test of their art. We have already spoken of the complicity of the phenomena in fermentations. Practical dyers endow the fermenting vat with a sort of personality. “An indigo vat,” says one to us, “is more like a sick man than any thing in the world: you have to watch it as you would a sick patient, and give it physic or ferments to stir up the system and purify it.” [5] The diagnosis of a sick vat requires that sort of instinctive knowledge which experience gives to the practised physician. The impatience of our young Americans will not permit them to serve the long apprenticeship necessary to acquire the proper experience. The artisans not thoroughly trained will naturally prefer the dyes and processes introduced by modern science, which require but little skill in their application. It is a curious fact that the influence of the national government has been largely instrumental in preserving the old system of indigo dyeing. Thanks to the Quartermaster-General’s Bureau, or the man of science, General Meigs, who presides over it, indigo dyed cloths have been persistently insisted upon for the army. The late war gave a new impulse to indigo dyeing. A skilled dyer, whom we have consulted, was constantly employed in Connecticut, on a tour of professional inspection of a dozen or more different establishments making army goods. No doctor, he says, ever found in hospital practice more complications of disease than he found in the 54 ailing vats. Among other difficulties there was a deficiency of imported woads, although the cultivation of excellent woad immediately sprung up in Connecticut. In the mean time carrot and rhubarb tops were used as substitutes for the fermenting material of the woad. Carrot-tops grown expressly for that purpose brought as high as twenty-five cents per pound. Since the war the requisitions for indigo dyed woollen goods have not relaxed, and the art is not likely to be lost.
With the real difficulties which attend the process, it is hard for indigo dyeing to sustain itself in the face of cheap substitutes of easy application, such as the Nicholson blue. It is exceedingly difficult to piece dye with indigo and preserve a uniform hue upon the cloth. Hence indigo dyes are generally given in the wool. The wool absorbing the foreign material of the dye is more difficult to work in the operations of carding and spining. In other words, a finer and costlier wool is required. A great desideratum therefore is a means of piece dyeing with indigo so as to preserve a perfect uniformity of hue throughout the piece. This, we are happy to say, has been recently successfully accomplished by one of the largest and most faithful of our cloth-making establishments. It would be premature, before the patents are secured for this invention, to explain the ingenious and expensive apparatus devised for this purpose, which constitutes in fact a battery of vats so arranged that the operation may be continuous. The experiments authorize the statement that bottom dyes of indigo, so desirable for a great variety of colors, can be applied with no other additional cost than that of the dyeing material. When this establishment, as it proposes, stamps upon the cards which designate goods, already so admirable in material and texture, “Warranted indigo dyed,” we shall regard it as an era in the American card-wool manufacture.
The old European woad vat process is that used in all our establishments. Mr. Henderson of the Washington Mills, whose experience as a practical dyer of wool is exceptionally large, informs us that he has found no work so instructive upon this process as Napier’s “Chemistry of Dyeing” (published by Henry 55 Carey Baird, of Philadelphia, 1869). Napier’s description of the process is extracted from Dumas’s “Lectures on Dyeing.” The appreciation expressed by so competent a judge induces us to reprint Dumas’s description in an appendix to this article.
That we may give at least a general view of the whole subject, we will proceed to consider indigo in some relations not yet adverted to.
In Part I. of our notes we have treated only of the application of this substance in dyeing by means of reduction through the indigo vat. Indigo may be applied by means of reduction in the printing of fabrics, as well as in dyeing them. A true scientific arrangement would compel us next in order to consider this other application of indigo by means of reduction. But the more natural and practical order is to pursue the subject of dyeing, and to consider next the applications of the derivatives from indigo in dyeing proper.
A reply by Mr. D. R. Whitney, an extensive indigo importer, to a letter of inquiry, enables us to correct some errors in our former article, under the head of “commerce in indigo.” The value of export from India in 1862–63, stated in dollars, through a typographical error, should have been pounds sterling; thus, instead of $2,126,814, read £2,126,814. It is stated in our first article that the telegrams show a decline of price of indigo in the Indian trade of from 50 to 75 per cent; “per cent” should read “rupees,” which would make a decline of from 25 to 30 per cent. The reason for the decline, as stated by Mr. Whitney, is the unusually large crop of this year. The average crop of indigo in Bengal is about 100,000 maunds. The crop of this year is 135,000 maunds, about 30 to 35 per cent above the average.
According to Mr. Whitney, the consumption of Bengal indigo in the United States was 2,458 cases of 270 lbs. to a case on an average, in 1871; and in 1872, 1,802 cases. Guatemala indigo, 3,132 serroons in 1871, and 2,578 serroons in 1872.
The powerful action of sulphuric acid upon indigo, and the bright and lively blue color thereby produced, had been observed by chemists long ago; but no person appears to have applied this color upon cloth, until it was done about the year 1740, by Counsellor Barth, at Grossenhein, in Saxony. The vividness of the dye, and the facility with which it was applied, brought it into great vogue under the name of Saxon blue, from its origin. Its popularity in former times is evinced by the words of the old song, “The Blue Bells of Scotland:”—
The Saxon blue consists simply of a solution of indigo, the Guatemala blue indigo being preferred, in sulphuric acid suitably diluted with water. The result of this reaction is not a single chemical substance, but two acids giving different tints, one called sulpho-purpuric acid or phenicine, and the other sulpho-indigotic acid; the first giving to wool a reddish-violet 56 color, and the other a pure blue. A third compound has been indicated by Berzelius, the nature of which has not been determined. Whether one or the other of the two named acids, or the two combined, shall be produced by the reaction between the sulphuric acid and the indigo, depends upon the duration of the contact, the temperature of the mixture, and the nature and proportion of the acid used.
Persoz gives the following general receipt:—
| 1 | part by weight of | indigo, finely rubbed. |
| 1 | „ „ „ „ | Nordhaussen acid. |
| 1 | „ „ „ „ | ordinary sulphuric acid. |
Leave for forty-eight hours, then heat until a drop turned into water will dissolve without producing a precipitate. Leave to cool, and dilute with water till the strength is brought to 18 Beaumé.”
Napier says that he has found the following method of preparing sulphate of indigo, in quantities for use, very satisfactory: “The indigo is reduced to an impalpable powder, and completely dried by placing it on a sand bath or flue for some hours at a temperature of about 150° F. For each pound of indigo six pounds of highly concentrated sulphuric acid are put into a large jar, or earthen pot, furnished with a cover. This is kept in as dry a place as possible, and the indigo is added gradually in small quantities. The vessel is kept closely covered, and care taken that the heat of the solution does not exceed 212°F. When the indigo is all added, the vessel is placed in such a situation that the heat may be kept up at about 150°F., and allowed to stand, stirring occasionally, for forty-eight hours. These precautions being attended to, we have uniformly found that any failure occurring was clearly traceable to the impurity of the indigo or weakness of the acid used.”
The processes for producing and separating the two acids derived from the combination of sulphur and indigo are minutely given by Berzelius, in vol. i. of his “Traité de Chemie,” who states this curious fact illustrative of the peculiar affinities of wool with certain dyeing substances. Wool or flannel thoroughly scoured, when immersed in the blue solution of indigo with 57 sulphuric acid, acts as a base: it combines gradually with the acid blue, and becomes itself colored of a deep blue. When saturated with color, it is withdrawn. Fresh wool is introduced until the bath yields no more color. If sublimed or perfectly pure indigo is used, there remains in the bath nothing but free sulphuric acid. The wool thus plays the part of a base with which the blue acids combine. The dyed wool is afterwards washed and treated in feeble alkaline bath (ammonia), which redissolves the blue. This method of purifying the Saxon blue is still practised by French manufacturers.
The combination of indigo with sulphuric acid, sometimes improperly called sulphate of indigo, is known by the dyers here and in England under the name of chemic. The name of chemic blue or green is also given the dyes formed from the indigo extract hereafter spoken of. It is largely used for making certain greens required in Scotch plaids.
The old Saxon blue or simple solution of indigo with sulphuric acid is now seldom prepared by the manufacturers themselves. It is now generally prepared for them, and furnished commercially under the name of indigo extract. The finer qualities used for fine dyeing and printing are known under the name of carmines of indigo, neutral extract, soluble indigo, ceruline, &c.
The production of indigo carmines, which are simply alkaline sulphindigotates or sulpho-purpurates, is founded upon their insolubility in a liquid charged with a salt.
If, for example, we dissolve one part of indigo in four parts of fuming acid, and dilute the liquid with sixty or eighty times its weight of water, it will contain, besides the sulphindigotic acid, an excess of sulphuric acid. By adding one part of crystals of soda so as to neutralize the bath, there will be formed not only sulphindigotate of soda, but sulphate of soda: as the former is insoluble in the saline liquid, the presence of the sulphate of soda causes the precipitation of the sulphindigotate in deep blue floccules. These are collected on woollen filters and washed to remove the sulphate of soda and a green coloring material, probably a modified chlorophyl, which the paste often contains, and which has the singular property of fixing itself on silk, but not on wool.
58
The carmines are divided according to their richness in indigo into simple carmine (4.96 per cent of indigo, water 89, saline materials 57), double carmine (10.2 per cent indigo, water 85, salts 4–8), triple carmine (12.4 per cent indigo water, 73.7, salts 13.9). A species of solid carmine known as Boiley blue or purple is in high repute in France.
The carmines may be tested by dyeing a specimen of wool in an acidulated bath to which cream of tartar has been added. The presence of the green matter, so objectionable to silk-dyers who make much use of these carmines, is detected by rubbing a small quantity of the carmine on a piece of glazed paper, which, when the color dries, gives a color varying from blue to a rich copper color: if any green coloring matter is left, it shows itself by a green aureola around the blue color. The method of applying the carmines in dyeing wool and silk,—for they are not adapted to cotton fabrics,—as given by M. de Kæppelin, is as follows:—
The operation is conducted in small wooden vats, provided with openings for manipulation, and pipes for inducting steam to heat the baths to the proper temperature. It consists of two parts, that of mordanting and dyeing. The former is thus conducted.
For each kilogram of tissue which has been previously scoured and bleached, there are provided 200 grammes of cream of tartar and 250 grammes of alum. These are dissolved in the bath of water of the vat, the temperature is raised to boiling heat, and the tissue is immersed in the bath f of an hour while it is worked over through the opening for manipulation. The pieces are then taken from the bath, to which is added a solution of the carmine in water containing a quantity of coloring matter proportionate to the intensity of the blue sought for. The solution ought to be prepared with care and passed through a silk sieve, so that the small insoluble grains which might have been left through bad fabrication may be left on the sieve. After the pieces have been manipulated in the colored bath, so as to exhaust the color and obtain the required blue, they should be rapidly washed in running water and dryed in the shade. Silk stuffs are dyed in the same way; 59 but the alum should be previously applied cold by means of a saturated solution of alum, in which the stuffs should be immersed for an hour.
In regard to all the combinations of indigo with sulphuric acid, including the carmines, it must be observed that their application does not constitute true indigo dyeing: the colors are not fast. It is not pure indigotine which is fastened on the tissues as in the vat dyeing, but another compound of indigo with the sulphur. Berzelius observes that “the color of soluble indigo is fully as alterable and fugacious as that of the colors extracted by the decoction of vegetable materials. By a long exposure to the sun the indigo blue is destroyed: it becomes green during evaporation, and changes its nature.” The carmines as well as the sulphur acids are easily decolorized by reducing agents, such as hydrogen and sulphuretted hydrogen, although they gradually assume their original color when exposed to the atmosphere. We are informed by some of the older dealers that imported cloths and merino stuffs known as “Saxony” were formerly largely sold in our shops, but that, notwithstanding their attractiveness to purchasers, they were objectionable on account of the instability of their color.
Our notes would be incomplete without some reference to the uses of indigo in printing fabrics. In pursuing this branch, we are embarrassed on the one hand by the consideration that the subject is too technical for the general reader, and on the other by the consciousness that it would be presumption in us to attempt to instruct those skilled in the art. It may not, however, be without benefit in producing a higher appreciation of science for the general reader to observe how science comes in play, even in the printing of a single color; while to the skilled reader our notes may possibly be of value as a vehicle for conveying some receipts taken from works not easily accessible.
60
This branch of our subject is directly allied to the one last considered, the application of the compounds of sulphur and indigo; for indigo is applied to printing wool and silk principally in the form of indigo carmines. These applications are less numerous than they were formerly, since they have been replaced by Prussian blue, and more recently by the aniline blues, which are now generally used. When the carmines are used, it is for making sky blues, and they enter into the composition of some greens and browns. The salts of alumina and vegetable acids are used to fix the indigo carmine upon tissues of wool and silk. Some receipts recommended by M. de Kæppelin, himself a practical printer, are given in a note. [7]
61
In printing tissues of wool with cotton warp, the carmines are not used alone. They are combined in certain proportions with cyanites of iron and potash, to obtain upon the cotton a blue color of equal intensity with that produced by the carmines upon wool. It is also necessary to previously mordant the fabrics by means of a solution of oxide of tin or caustic soda which is precipitated on the fibres by passing through a bath of water, to which sulphuric acid has been added.
| BLUE NO. 1. | ||||
| Indigo carmine | 400 | grammes. | ||
| Alum | 100 | „ | ||
| Oxalic acid | 150 | „ | ||
| Boiling water | 1¼ | litre | ||
| Gum water prepared in proportion of 1 kilogram to the litre | 1¼ | litre | ||
| GREEN NO. 1. | ||||
| Gum water as above | 12 | litres. | ||
| Cuba lac | 12 | „ | ||
| Alum | 1 | kilogram, | 500 | grammes. |
| Oxalic acid | 2 | „ | ||
| Indigo carmine | 4 | „ | ||
| BOUILLON FOR THE GREENS AND BLUES. | ||||
| Boiling water | 12 | litres. | ||
| Alum | 600 | grammes | ||
| Oxalic acid | 750 | „ | ||
| Gum water | 12 | „ | ||
| SKY BLUE FOR WOOLLEN STUFF WITH COTTON WARP. | ||||
| First solution.—Boiling water | 4 | litres. | ||
| Cyanuret of iron and potash | 800 | grammes. | ||
| Second solution.—Boiling water | 2 | „ | ||
| Tartaric acid | 300 | „ | ||
| Third solution.—Cold water | 3 | „ | ||
| Sulphuric acid | 300 | „ | ||
| Pour in the first solution, then the second and third, agitating the color with a spatula after each new addition. | ||||
| The following mixture is afterwards applied to the stuff:— | ||||
| Gum water | 12 | litres. | ||
| Water | 6 | „ | ||
| Blue No. 1 for wool | 3 | „ | ||
Before entering upon methods used in large establishments, it may not be without interest to observe the processes still used in Java for printing calicoes, which the natives prefer to any imported from Europe. In Java there are no factories, and the women in each family make and dye or print all the cotton cloths required for their own consumption. They apply by means of a brush or pencil, which they use with great skill, to the cotton tissue which they wish to cover a thin coating of wax mixed with a little resin, the wax being applied to all the parts where the design, which has been first traced upon the cloth, requires that the fabric should remain uncolored. They then immerse the stuff several times in an indigo vat until they have obtained the desired tint. The stuff is afterwards washed and dried for a new application of the wax, carefully applied with a pencil as before. The cloth is then immersed in a bath of a different color, made with madder or catechu, but always of some dye which is perfectly stable; and the operation is repeated according to the number of colors desired. By these successive applications of wax and immersions into different vats, they succeed in producing very complicated and harmonious colors, while no European goods compare with them in stability of dye.
In the European, and our own manufacture, the blue bottoms upon vegetable fibres, made by immersion in the indigo vat, are combined with white impressions, or others variously colored, by two distinct methods. Sometimes there is printed upon the cloth before dyeing in the indigo vat a preparation called a reserve or resist, which prevents the indigotine from being deposited in the places where it is applied. Sometimes, on the 62 contrary, the indigo, which has been uniformly fixed upon the fabric, is destroyed in certain places marked out by printing upon them certain chemical agents, called discharges.
The reserves are mechanical, resisting the penetration of the dye, such as wax and pipe clay, or chemical. The last, through these acid or oxidizing properties, cause the precipitation of the indigotine before it has touched the fibre or penetrated into its pores. Such are the salts of copper and bi-chlorate of mercury. Other bodies perform the part both of mechanical and chemical reserves. The salts of zinc or alumina, for instance, which are frequently used, produce at the same time a deposit of indigo white and a gelatinous covering of hydrated oxide of zinc or aluminium. The composition of a good reserve is declared to be principally a question of good proportions of the constituent parts, varying with the strength of the vat and the intensity of the blue which is desired to be reserved. The first condition is that it hardens immediately after immersion in the vat: if it softens, on the contrary, it will cause the running of the color. In other words, the acidity of the impression should be proportionate to the strength and alkaline character of the vat. The white reserve, that most generally used, is composed of pipe clay, gum, verdigris, and sulphate of copper. The styles of work produced by dipping with reserves are generally of a cheap and low class. The system is clumsy and expensive, and is only tolerated because of the want of a method of directly applying indigo, which will yield the deepest shades.
Certain styles, formerly in great vogue, called Lapis, and forming one of the richest branches of the cotton-printing industry, are founded upon the use of reserves; and in these styles, by very simple means which we shall not attempt to describe, different colors produced from madder, catechu, &c., are produced upon the fabric so perfectly surrounded by blue that the eye cannot detect the slightest want of continuity. This fabrication has the greatest perfection in Russia. The imitation cashmere fabrics of cotton imported from that country, formerly much in fashion for dressing-gowns, are specimens of this fabrication. The great stability of the colors is a remarkable feature of these goods.
63
The system of resists or reserves possesses the inconveniences of not producing impressions of great firmness, and of requiring very strong vats. When the strength of the vat is partially exhausted, they may be thrown aside. These inconveniences are obviated by the system of discharges (enlevages). In this system the cloths are vat dyed of a uniform blue. The strength of the vat is of less importance, and it can be used until the indigo is quite exhausted. The means of destroying the indigo which has been fixed upon the fibre are founded on the use of active oxidizing agents, which transform the insoluble indigotine into soluble isatine. The agent generally used is chromic acid. As this acid cannot be incorporated with the thickening to be printed, as the thickening would produce oxide of chrome, the cloth is passed through a strong solution of chromate of potash, and dried in the shade. The required pattern is then printed on the cloth with a mixture whose principal elements are acids which are susceptible of setting free the chromic acid on the tissue, which then acts upon the indigo producing a white pattern. The acid generally employed for freeing the chromic acid is oxalic acid, thickened with British gum, dextrine, or starch, with the addition of pipe clay. To prevent running, nitric, sulphuric, or tartaric acid are sometimes used. [8]
64
By the method of discharges the white designs upon blue are brought out with a distinctness which it is impossible to obtain by resists, while the most delicate work of the graver can be exactly reproduced upon the tissue.
Schutzenberger gives the following receipts:—
| PREPARATION FOR DISCHARGE. | |
| Water | 2 litres. |
| Yellow chromate | 500 grammes. |
| WHITE DISCHARGE ON BLUE BOTTOM. | |
| Tartaric acid | 3 kilograms. |
| Oxalic acid | 250 grammes. |
| Burnt starch | 4 kilograms. |
| Nitric acid | 500 grammes. |
| Water | 4 litres. |
De Kæppelin gives the following:—
| WHITE DISCHARGE ON BLUE. | |
| Water | 2 litres. |
| Starch | 1 kilogram, 800 grammes. |
| Oxalic acid | 500 grammes. |
| Tartaric acid | 250 grammes. |
| Sulphuric acid | 375 grammes. |
The pieces, having been dyed blue, are then placed in a solution of bichromate of potash in water, which is prepared in the ratio of 50 to 60 grammes to the litre, according to the intensity of the blue. The pieces thus prepared must be dried away from direct solar light or too much heat. In fact, under the action of these agents, the bichromate would be decomposed and the tissue altered. The pieces are often rolled up to prevent this effect. After the pieces are printed, they are passed into a vessel containing water and holding chalk in suspension in sufficient quantity to give it a milky aspect. The temperature of the bath is raised to 60° R. The excess of acid of the color applied is saturated by the chalk, and the excess of bichromate of potash with which the tissue is impregnated is dissolved in the bath. The pieces are afterwards washed and passed through slightly soapy water.
The first step in the art of printing indigotine upon calicoes was the application of what is called pencil blue. Instead of immersing the fabrics in an indigo vat, the indigo white formed in a very strong indigo vat was thickened and applied locally to certain places on the cloth. The preparation was painted upon the cloth by means of pencils made of willow sticks, the ends of which were broomed up into a kind of brush. The style was hence called pencil blue. The methods now used to apply white indigo locally are of two kinds. The china blue process, and the solid blue process, sometimes called fast or precipitated blue. The china blue process derives its name from the resemblance of its color to the blue on the old china ware. It has great depth of tint, and permanency. It is scarcely used now, except for certain articles requiring great depth of color, such as certain furniture goods, and by the Germans and Swiss for the manufacture of calicoes for exportation to India.
We do not venture to condense the descriptions at our hand of the processes for applying the china blue and the solid blue, and translate those furnished by chemists of high authority. After the method indicated by Darwin in his recent works, we present them in smaller type, with the perhaps unnecessary suggestion that they may be passed over by the general reader.
China blue.—The theory of this printing blue, says Schutzenberger, is very simple. The indigo, reduced to an impalpable powder and 65 thickened, is printed by a plate or roller. After drying, the tissue seems dyed blue, more or less deep, according to the proportion of coloring material used; but it is only a blue of application, which can be removed with the thickening, by the slightest washing. The object is now to reduce and redissolve the indigotine in place to enable it to penetrate the fibre at the end of a consecutive oxidization, and without producing a running of the color or altering the purity and distinctness of the contours of the design. I owe to M. Ed. Schwartz some valuable hints upon the fabrication of this style, which is also described with much care and details in the treatise on printing by M. Persoz.
The reduction of the indigo is obtained by alternate passages of the printed tissue into vats containing,—the first, quicklime slacked; the second, sulphate of iron; the third, soda. The operation is terminated by a passage through a bath of sulphuric acid, which removes the oxide of iron and precipitates the indigo white by hastening its oxidation.
The success depends upon the composition of the color printed, and above all upon the strength of the vats of immersion and the duration of the treatment.
The operator uses six vats,—for instance, two lime vats, provided each with 12 kilograms of lime; a copperas vat at 70 Beaumé; a caustic soda vat marking 140 Beaumé; a sulphuric acid vat with 500 grammes of acid (par mesure d’eau); and finally a vat of pure water.
The receipts for printing are:—
| 1. THE BLUE PREPARATION. | ||
| Ground indigo | 4 | kilograms. |
| Acetate of iron | 10 | litres. |
| Sulphate of iron | 1 | kilogram. |
| Water | 10 | litres. |
| Gum Senegal | 6 | kilograms. |
Pass through a sieve; leave some time at rest, and stir whenever used. Caraccas indigo is preferred because it can be broken into a finer powder and gives a finer paste.
| 2. COLORS FOR ROLLER PRINTING NOS. 1, 2, 3, 4. | |
| The blue preparation above | 1, 1, 3, 4. |
| Acetate of iron containing 700 grammes of gum per litre | 2, 1½, ½, ½. |
| Gum water at 600 grammes per litre | 16, 2½, ½, ½. |
These proportions can be varied according to the tint desired.
66
The piece is treated a quarter of an hour in the first lime vat by giving it a light movement from above to below; it is left a quarter of an hour in repose in the sulphate of lime vat; a quarter of an hour in the second lime vat; a quarter of an hour in the copperas vat; five minutes in the caustic soda; half an hour in the sulphuric acid, and then thoroughly rinsed.
To each lime vat there is given 2 kilograms of lime per piece of cloth. To the vitriol vat there is added 50 kilograms of sulphate of iron for each dozen pieces. The soda vat is renewed after 5 pieces by the addition of 12 kilograms of salt of soda, which has first been made caustic. The acid vat receives 25 kilograms of acid after 5 pieces, and ought to be renewed whenever it becomes saline. The other vats must be cleared out whenever the deposit becomes too great for success.
M. Ed. Schwartz recommends as important conditions, (1) the perfect causticity of the tissue, and an average strength of 140 Beaumé; (2) the neutrality of the sulphate of lime vat. For this end old iron should be boiled in it.
After leaving the sulphuric acid vat the pieces are rinsed in the water vat, then in river water, and afterwards should be soaked in a sulphuric acid bath at 40 Beaumé, for the purpose of dissolving the last traces of the peroxide of iron adhering to the fibre. The fabric is then washed in water and finally passed through a soapy water at 40° R.
Solid or precipitated blue, Schutzenberger’s receipt.—The process consists in printing indigo white precipitated in a vat, in a thick paste to dissolve it on the tissue by a passage through an alkaline bath (lime or soda), and of reprecipitating it by oxidizing it as soon as it has entered the fibre.
It is then the china blue process, minus the reduction which is made before printing, and consequently minus the sulphate of iron vat.
Indigo white is too alterable to be printed with success, so it is generally precipitated in combination with a stannic hydrate (hydrate of a salt of tin), which gives it body and preserves it from a too rapid oxidation.
The stannic indigotate in paste, or as it is generally called precipitate of indigo, is prepared by turning into the clear portion of a strong copperas vat an acid solution of protochlorate of tin, and filtering it upon woollen filters,—as much as possible away from the air. 67 It would be better to prepare a strong tin vat by heating a mixture of indigo, caustic soda, and protochlorate of tin, and to precipitate by chlorohydric acid. [9]
The deposit is made into a paste with gum water; a salt of tin is often added to prevent oxidation. It is important to prevent the transformation of the indigo white into indigotine before printing. This indigotine would not fix itself on the fabric. Moreover, after printing, it is necessary to hasten the dissolution of the indigo white to enable it to penetrate the fibre. It is sufficient for this end to pass it through milk of lime. The stannic combination is immediately destroyed; the colorable matter unites itself with the lime, and the color passes into a pale gray with apple green. The indigo white becomes momentarily soluble; but the presence of the excess of lime and the thickening, as well as the attractive affinity of the thickening, prevent any running.
The piece on issuing from the lime water is placed in running water, when reoxidation commences, which this time fixes the color. The piece is finally passed through a sulphuric acid bath to absorb the lime, and washed.
By adding to the color a salt whose base precipitates in the milk of lime and oxidizes in the running water, and replacing the simple acid bath by an acid bath with yellow prussite, the intensity of the blue is increased through the formation of Prussian blue.
68
Although we have seen beautiful effects from the application of the solid blue of indigo on prints at our Pacific Mills, the colors produced by Prussian blue and aniline are so much more brilliant and easy of application that the use of indigo in printing goods for ordinary consumption is likely to decline rather than increase. It will be otherwise if we should ever manufacture for the East India markets. Here is a field still open for our manufacturers. Mr. Watson, in his beautiful work on “The Costumes of the People of India,” remarks that “British manufacturers have hitherto failed to appreciate Oriental tastes and habits, and hence supply but an insignificant part of the clothing of the two hundred million persons that form the population of what is commonly spoken of as India.” The great defect, he observes, is the want of stability of color in the cotton fabrics introduced,—this stability being an imperative demand in the Oriental markets.
The applications of indigo to cotton fabric are altogether secondary, in our mind, to its relations to the woollen manufacture. If we have felt called upon to say a word in behalf of the most ancient and best ally which the fibre of wool has ever had, it is because the vividness of color of the new products of coal, and the fascination which the application of the recent discoveries of science always possesses, is threatening the eclipse of the more ancient sober and solid dyes. Let the new colors have their place as auxiliaries, not as substitutes for the ancient dyes. Let them serve to give a bloom [10] to goods, but let the foundation be the good old dyes which the experience of ages has proved to be the most unalterable by light and air. The recent wonderful discovery of alizarine, or artificial madder, in coal tar products, has led practical men to expect too much from science. The opinion is quite prevalent among manufacturers that artificial indigotine has already been obtained from the 69 same source. And some manufacturers are sanguine that the difficulties of indigo dyeing will thus be resolved. It is not improbable—for what is impossible to modern chemistry?—that this result will yet be partially obtained. But we have looked over all the recent foreign chemical reviews, and personally consulted some of our best chemists, and we can find no authority for the prevailing opinion that artificial indigotine has been produced. If the production of artificial indigotine should be realized, the only benefit would be the possible cheapening of the material. The difficulties of the indigo vat would still remain; for we cannot too often repeat, that in the very difficulties of the process, or in the insolubility of blue indigotine by ordinary agents, consists the excellence of the dye.
Mr. T. P. Shepard gives in his valuable “Receipts for Calico Printing,” published in 1872, the following:—
NO. 52. INDIGO PRECIPITATE FOR FAST BLUE AND GREEN.
The indigo precipitate is to be collected on a muslin filter, and well squeezed out.
Guernsey Blue.—The darkest of the Nicholson Fast Blues. On a bottom of barkwood, camwood, madder, or inferior indigo, produces an indigo blue which will stand all the acid tests the same as colors made from indigo.
Serge Blue.—It will be found very serviceable to give bloom to goods dyed with indigo, and by itself shows a very good indigo test with nitric acid.—Instructions for Working the Atlas Works Aniline Dyes.
[Extract from Dumas’s Lectures on Dyeing.]
The value attached by practical wool-dyers to the following induces us to publish it without condensation:—
Indigo Blue.—We give a solid dye of indigo blue to wool by plunging it into an alkaline solution of indigo white, and then exposing it to contact with the air. The solution of indigo white is prepared in a vessel usually from eight to nine feet in depth, and six to seven feet in diameter. This size is very convenient for the requisite manipulations, and presents a large volume of water, which, when once heated, is capable of preserving a high temperature for a long time. This vessel should be made of wood or copper. It always bears the name of vat. These vats are covered with a wooden lid, divided into two or three equal segments. Over this lid are spread some thick blankets. Without this precaution the bath would come in contact with the atmospheric air, a portion of the indigo would absorb oxygen and become precipitated. There would also be a great waste of heat.
A most necessary operation, and one which has to be frequently repeated, consists in stirring up the deposit of vegetable and coloring matter which is formed in the vat, and intimately mixing it in the bath. For this purpose we employ a utensil called a rake, which is formed of a strong square piece of wood, set on a long handle. The workman takes hold of this with both hands, and, dipping the flat surface into the deposit at the bottom of the vessel, he quickly 70 draws it up until it nearly reaches the surface, when, giving it a gentle shake, he discharges the matter again through the liquor of the bath. This manœuvre is repeated until the whole of the deposit seems to be removed from the bottom of the vessel. Before the tissue is dipped into the dye-bath, it should be soaked in a copper full of tepid water; it is then to be hung up and beaten with sticks. In this state it is plunged into the vat; it thus introduces less air into the bath, while it is more uniformly penetrated by the indigo solution. The cloth is now kept at a depth of from two to three feet below the surface of the liquid, by means of an open bag or piece of network fixed in the interior of an iron ring, which is suspended by cords, and fixed to the outside of the vat by means of two small iron hooks; the bag is thus drawn backwards and forwards without permitting it to come in contact with the air. When this operation has been continued for a sufficient length of time, the cloth is wrung and hung up to dry.
Flock wool is also, for the purpose of dyeing, enclosed in a fine net, which prevents the least particle from escaping, and which is fixed in the bath in the same way as in the foregoing case.
The many inconveniences attending the use of wooden baths, which necessitate the pouring of the liquor into a copper for the purpose of giving it the necessary degree of heat, have led to the general employment of copper vessels. These are fixed in brickwork, which extends half way up their surface, whilst a stove is so constructed at this elevation that the flame shall play around their upper part. By this means the bath is heated and kept at a favorable temperature without the liquor being obliged to be removed.
The potash vats are usually formed of conical-shaped coppers, surrounded by a suitable furnace. These may be constructed with less depth, inasmuch as there is less precipitation induced in the liquor. By using steam for heating the vats, we might dispense with the employment of copper vessels, and so return to those of wood.
The vats employed for dyeing wool are known under the names of the pastel vat, the woad vat, the potash vat the tartar-lee vat, and the German vat.
Pastel Vat. [11]—The first care of the dyer in preparing the vat should be to furnish the bath with matters capable of combining with the oxygen, whether directly or indirectly, and of giving hydrogen to the indigo. We must, however, be careful to employ those substances only which are incapable of imparting to the bath a color which might prove injurious to the indigo. These advantages are found in the pastel, the woad, and madder. This latter substance furnishes a violet tint when brought into contact with an alkali, and by the addition of indigo it yields a still deeper shade.
In preparing the Indian vat, we ordinarily employ one pound of fine madder to two pounds of indigo. The madder is here especially useful, by reason of the avidity of some of its principles for oxygen.
The pastel vat, when prepared on a large scale, ordinarily contains from 18 to 22 lbs. of indigo; 11 lbs. of madder would suffice for this proportion, but we must also bear in mind the large quantity of water which we have to charge with oxidizable matters. I have invariably seen the best results from employing 71 22 lbs. to a vat of this size. Bran is apt to excite the lactic fermentation in the bath, and should therefore not be employed in too large a quantity; 7 to 9 lbs. will be found amply sufficient.
Weld, which is often used, is rich in oxidizable principles; it turns sour, and passes into the putrid fermentation with facility. Some dyers use it very freely; but ordinarily we employ in this bath an equal quantity of it to that of the bran. Sometimes weld is not added at all.
In most dye-houses the pastel is pounded before introducing it into the vat. Some practical men, however, maintain that this operation is injurious, and that it interferes with its durability. This is an opinion which deserves attention. The effect of the pastel, when reduced to a coarse powder, is more uniform; but this state of division must render its alterations more rapid. When the bath has undergone the necessary ebullition, the pastel should be introduced into the vat, the liquor decanted, and at the same time 7 or 8 lbs. of lime added, so as to form an alkaline lye which shall hold the indigo in solution. Having well stirred the vat, it should be set aside for four hours, so that the little pellets shall have time to become thoroughly soaked, both inside and out, and thus be prepared for fermentation. Some think coverings are to be spread over the vat, so as to preserve it from contact with the atmosphere. After this lapse of time, it is to be again stirred. The bath at this moment presents no decided character; it has the peculiar odor of the vegetables which it holds in digestion; its color is of a yellowish-brown.
Ordinarily, at the end of twenty-four hours, sometimes even after fifteen or sixteen, the fermentative process is well marked. The odor becomes ammoniacal, at the same time that it retains the peculiar smell of the pastel. The bath, hitherto of a brown color, now assumes a decided yellowish-red tint. A blue froth, which results from the newly liberated indigo of the pastel, floats on the liquor as a thick scum, being composed of small blue bubbles, which are closely agglomerated together. A brilliant pellicle covers the bath, and beneath we may perceive some blue or almost black veins, owing to the indigo of the pastel which rises towards the surface. If the liquor be now agitated with a switch, the small quantity of indigo which is evolved floats to the top of the bath. On exposing a few drops of this mixture to the air, the golden-yellow color quickly disappears, and is replaced by the blue tint of the indigo. This phenomenon is due to the absorption of the oxygen of the air by the indigogen from the pastel: in this state we might even dye wool with it without any further addition of indigo; but the colors which it furnishes are devoid of brilliancy and vivacity of tone, at the same time that the bath becomes quickly exhausted.
The signs above described announce, in a most indubitable manner, that fermentation is established, and that the vat has now the power of furnishing to the indigo the hydrogen which is required to render it soluble,—that contained in the pastel having been already taken up; this, then, is the proper moment for adding the indigo, which should be previously ground in a mill.
We stated above that the liquor of the vat should be previously charged with a certain quantity of lime; we also find in it ammonia generated by the pastel; but a part of these alkalies become saturated by the carbonic acid gas along with the proper acids of the madder and of the weld, as well as by the lactic acid produced by the bran during fermentation. The ordinary guide of the dyer is the odor, which, according to circumstances; becomes more or less ammoniacal. The vat is said to be either soft or harsh; if soft, a little more lime should be added to it. The fresh vat is always soft; it exhales a feeble 72 ammoniacal odor accompanied with the peculiar smell of the pastel; we must, therefore, add lime to it along with the indigo; we usually employ from five to six pounds, and, after having stirred the vat, it is to be covered over. The indigo, being incapable of solution except by its combination with hydrogen, gives no sign of being dissolved until it has remained a certain time in the bath. We may remark that the hard indigoes, as those of Java, require at least eight or nine hours, whilst those of Bengal do not need more than six hours, for their solution. We should examine the vat again three hours after adding the indigo. We ordinarily remark that the odor is by this time weakened; we must now add a further quantity of lime, sometimes less, but generally about equal in amount to the first portion; it is then to be covered over again, and set aside for three hours.
After this lapse of time, the bath will be found covered with an abundant froth and a very marked copper-colored pellicle; the veins which float upon its surface are larger and more marked than they were previously; the liquor becomes of a deep yellowish-red color. On dipping the rake into the bath, and allowing the liquid to run off at the edge, its color, if viewed against the light, is of a strongly marked emerald-green, which gradually disappears, in proportion as the indigo absorbs oxygen, and leaves in its place a mere drop rendered opaque by the blue color of the indigo. The odor of the vat at this instant is strongly ammoniacal; we also find in it the peculiar scent of the pastel. When we discover a marked character of this kind in the newly formed vat, we may without fear plunge in the stuff intended to be dyed; but the tints given during the first working of the vat are never so brilliant as those subsequently formed; this is owing to the yellow-coloring matters of the pastel, which, aided by the heat, become fixed on the wool at the same time as the indigo, and thus give to it a greenish tint. This accident is common both with the pastel and the woad vats; it is, however, less marked in the latter.
When the stuff or cloth has been immersed for an hour in the vat it should be withdrawn; it would, in fact, be useless to leave it there for a longer time, inasmuch as it could absorb no more of the coloring principle. It is therefore to be taken from the bath and hung up to dry, when the indigo, by attracting oxygen, will become insoluble and acquire a blue color. Then we may replunge the stuff in the vat, and the shade will immediately assume a deeper tint, owing to renewed absorption of indigo by the wool. By repeating these operations, we succeed in giving very deep shades. We must not, however, imagine that the cloth seizes only on that portion of indigo contained in the liquor required to soak it. Far from such being the case, experience shows that, during its stay in the bath, it appropriates to itself, within certain limits, a gradually increasing quantity of indigo. We have here, then, an action of affinity, or perhaps a consequence of porosity on the part of the wool itself.
Woad Vat.—These vats are extensively employed at Louviers, and in the manufactories of the north of France. The bath is prepared in the same manner as in the foregoing case; the finely cut root is introduced into the copper along with 2 lbs. of pounded indigo, 9 lbs. of madder, and 15½ lbs. of slaked lime. The liquor is, after the necessary ebullition, poured upon the woad. This substance contains but a very small quantity of coloring principle; we must, therefore, add some indigo when preparing the vat, so as to indicate the precise instant when the mixture arrives at the point of fermentation so necessary for imparting hydrogen to the coloring principle, and for rendering it soluble. We must also use a larger quantity of lime, since the woad contains no ammonia 73 resulting from previous decomposition, such as we find to be the case with the pastel of the south. When the vat is in a suitable state of fermentation, a rusty color becomes manifest, in addition to the signs already described in speaking of the pastel vat; besides the ammoniacal odor, the bath always retains the peculiar smell of the woad. The pounded indigo is now added, and we proceed, in the manner already detailed, to reduce it to a state of solution fit for dyeing.
The vats prepared by means of pastel have greater durability than those made with the woad; but it is thought that the colors given by the latter are more brilliant than those obtained from the former dye.
Modified Pastel Vat.—This vat is about 7 feet in depth, and 6½ feet in diameter. It is made of copper, and heated by steam. The lid is composed of three segments, each of which is formed of two planks, about an inch thick, and strongly secured together by bolts.
The beating is performed in the usual way, with sticks before the first dipping, after having moistened the cloth in tepid water. This operation is not subsequently repeated.
This vat is prepared with 13 lbs. of indigo, 17½ lbs. of madder, 4½ lbs. of bran, 9 lbs. of lime, and 4½ lbs. of potash. Having filled the vat, we heat it to about 200° Fah., and, as soon as the water is tepid, introduce 441 lbs. of pastel. The liquor becomes of a yellowish-brown color; small bubbles appear upon its surface, ordinarily at the end of four hours if the vat be heated by steam, but not until after eight or twelve hours where heat is applied by the common fire; in the latter case the mixture should be stirred every three hours. When the liquor displays the signs of fermentation, we add the above-mentioned ingredients, and cover the vat over; it is then to be set aside, stirring it every three hours, or oftener if the fermentative action be very rapid. Each time that it is stirred we are to add from 2 to 4 lbs. of lime; if fermentation proceed quickly we even use more, but in the contrary case less. After about eighteen hours, we plunge into the vat three pieces of common cloth, measuring from twenty to twenty-five ells in length each; when they have received six or seven turns, they are to be taken out again. The object of this is to remove the excess of lime from the bath. The vat is then set aside for three hours, when it is to be stirred, and 13 lbs. of indigo, with 2 lbs. of madder, added to it. We now again apply heat to the mixture.
If the vat contains a superabundance of lime, it will be unnecessary to add more; otherwise we throw in a further quantity. During the night it should be covered with a cloth, and a workman left to watch it. It is usually stirred once before the morning; but if it be deficient in lime, it will require this manipulation to be more frequently repeated, and also fresh lime added to it. On the following day the stirring should be continued every three hours, and so on for the next thirty hours, taking care to heat the vat from time to time. On the morning of the fourth day the dyeing may be commenced.
The temperature should be maintained at a pretty uniform point; if it be too hot, the blue takes a red reflection, by reason of the madder contained in the liquid. A vat thus prepared will last three months; we may even work it for double that period, but after the third month it appears to lose some of its indigo.
We maintain the power of the vat by introducing every night 2¼ lbs. of madder. Some indigo is also added twice or three times a week. These additions are made in the evening. After the former, the vat is left at rest for forty-two hours; with the latter only for twenty-four, at the same time observing 74 the precautions already indicated. At the end of three months, or sooner when we wish to stop the working of the vat, we exhaust the indigo; for this purpose we continue to charge it every night for the space of a month with madder, and dip into it white cloths, or more particularly woollen tissues, which become more or less loaded with the indigo. We must continue this plan until these matters take up no further color. The dippings are to be performed twice a day at first, but once only towards the termination. Many dyers make use of this bath for preparing a new vat, but it is better to throw this away and make it up afresh with common water.
Indian Vat.—These vats are of more simple and of more ready construction than the pastel or woad vats. We are to boil in water a quantity of madder and of bran, proportioned to the weight of indigo which we wish to employ. After two hours’ ebullition, we turn into this bath some tartar-lees, which are also to be boiled for an hour and a half or two hours, so as to charge the bath with whatever soluble matter they may contain; after this ebullition the bath should be allowed to cool, and the indigo, which has been previously ground, is then to be introduced. Supposing that we wish to employ 21 lbs. of indigo, the following would be the proportions used in preparing this vat: 41 lbs. tartar-lees, 13 lbs. of madder, and 5 lbs. of bran. These vats are usually mounted in coppers of a conical shape; a small fire should be kept up around them, so as to maintain a moderate and uniform heat. The indigo will usually be found dissolved at the end of twenty-four hours, often even after twelve or fifteen hours. The liquor has a reddish color in the new vats, and a green tint in those which are in a working state. The frothy surface, as well as the brilliant-colored pellicle, becomes manifested in this as in all other preparations of a like kind.
This species of vat has to be renewed much more frequently than the woad and pastel vats, from the indigo being more difficult to dissolve after a certain lapse of time. A moderate heat should be maintained in all these vats.
Potash Vat.—This species of vat is extensively employed at Elbœuf for the dyeing of wool in the flock. It presents in all respects a perfect analogy with the Indian vat; in fact, the action of the tartar-lee in the latter preparation depends entirely on the carbonate of potash which it contains. The ingredients used in the preparation of the potash vat are bran, madder, and the subcarbonate of potash of commerce.
We obtain the deep shades in this species of vat with greater celerity than in all others, a fact which undoubtedly depends on the greater power which potash has of dissolving indigo than is possessed by lime. Experience proves that the potash vat has the advantage in point of celerity of nearly a third; but this is balanced by the inconvenience resulting from the darker shade, which we must attribute to the large quantity of coloring matter of the madder dissolved by the alkaline lee, and which becomes fixed on the stuff with the indigo.
To render this vat in its most favorable state, the indigo should be made to undergo a commencement of hydrogenation before turning it into the mixture; for this purpose we prepare in a small copper a bath analogous to that in the vat, to which the pounded indigo is added. This bath is maintained for twenty-four hours at a moderate heat, taking care to stir it from time to time. The indigo assumes a yellowish color, becomes dissolved, and in this state is turned into the vat; we thus avoid many delays and losses in its preparation, and indeed it would be desirable if a similar plan were adopted with all these compounds.
German Vat.—This vat is of nearly similar dimensions to that used for the woad, being three times the size of the potash vat. Its diameter is about 6½ feet, 75 and its depth 8½ feet. Having filled the copper with water, we are to heat it to 200° Fah.; we then add 20 pailsful of bran, 22 lbs. of carbonate of soda, 11 lbs. of indigo, and 54 pounds of lime, thoroughly slaked, in powder. The mixture is to be well stirred, and then set aside for two hours; the workman should continually watch the progress of the fermentation, moderating it more or less by means of lime or carbonate of soda, so as to render the vat in a working state at the end of twelve, fifteen, or, at the most, eighteen hours. The odor is the only criterion by which the workman is enabled to judge of the good state of the vat, he must therefore possess considerable tact and experience.
In the process of dipping we introduce 84 lbs., 106 lbs., or even 130 lbs. of wool, in a net bag, similar to that used in the woad vat, taking care that the bag is not allowed to rest against the sides of the copper. When the wool has sufficiently imbibed the color, we remove the bag containing it, and allow it to drain for a short time over the vessel. We operate in this way on two or three quantities in succession; we then remove the vat, and set it aside for two hours; we must be careful, from time to time, to replace the indigo absorbed by the wool, as also to add fresh quantities of bran, lime, and crystallized carbonate of soda, so as constantly to maintain the fermentation at a suitable point.
The German vat differs, then, from the potash vat by the fact that the potash is replaced by crystallized carbonate of soda and caustic lime, which latter substance also gives to the carbonate of soda a caustic character. It presents a remarkable saving as compared to the potash vat; hence the frequency of its employment; but it requires great care, and is more difficult to manage. It also offers considerable economy of labor; one man is amply sufficient for each vat.
The army cloth is usually dyed by means of the pastel vat, which gives the most advantageous results. We here make use of vats about 8½ feet in depth, and 5 feet in diameter, into which we introduce from 361 lbs. to 405 lbs of pastel or of woad, after previous maceration. The vat is to be filled with boiling water, and we then add to the bath 22 lbs. of madder, 17½ lbs. of weld, and 13 lbs. of bran. The mixture is to maintained in a state of ebullition for about half an hour; we next add a few pailsful of cold water, taking care, however, not to lower the temperature beyond 130° Fah.; during the whole of this time a workman, provided with a rake, keeps incessantly stirring the materials of the bath. The vat is then accurately closed by means of a wooden lid, and surrounded by blankets, so as to keep up the heat. It is now put aside for six hours; after this time it is again stirred by means of a rake, for the space of half an hour; and this operation should be repeated every three hours until the surface of the bath becomes marked with blue veins; we then add from six to eight pounds of slaked lime.
The color of the vat now borders on a blackish-blue. We immediately add the indigo in a quantity proportioned to the shade which we wish to obtain. The pastel in the foregoing mixture may last for several months; but we must renew the indigo in proportion as it becomes exhausted, at the same time adding both bran and madder. In general we employ—
Management of the Vats.—A good condition of the vat is recognized by the following characters: The tint of the bath is of a fine golden-yellow, and its surface is covered with a bluish froth and a copper-colored pellicle. On dipping the rake into the bath, there escapes bubbles of air, which should burst very slowly; 76 when they vanish quickly, it becomes an indication that we must add more lime. The paste which is found at the bottom of the vat, green at the moment of its being drawn up, should become brown in .the air; if, however, it remains green, this is a further sign that more lime is required. Lastly, the vat should exhale the odor of indigo. We usually complete the assurance of the vat being in a good state by plunging into it, after two hours’ respite, a skein of wool, which, on being withdrawn after the lapse of half an hour, should present a green color, but change directly to blue. We then once more mix the materials of the vat, and two hours after it may be considered ready for dyeing.
These vats, like those already described, are provided with a large wooden ring, the interior of which is armed with a kind of network, for the purpose of preventing the objects which are intended to be dyed coming in contact with the materials at the bottom of the vat; we, moreover, take the precaution of enclosing the wool or cloth in bags. These tissues, when plunged into the bath, should remain there for a longer or shorter time, according to the shade which we wish to obtain; one dipping, however, will never suffice for this object; usually we leave in the stuff for half an hour only; it is then to be taken from the bath, wrung, and exposed to the air. This operation is repeated until we have succeeded in procuring the desired shade; we ordinarily suffer three hours to elapse between each dipping. The heat of the vat should never be allowed to fall below 130° Fah. After each operation the bath must be well stirred, and fresh lime added; generally speaking, a pound a day will suffice. We re-establish the indigo about every second day. When once this vat is well mounted, and we are careful to examine its working, we may dye from two to four batches a day with it.
When the stuffs have acquired the desired shade, they are first to be washed in common water, and then in a very weak solution of hydrochloric acid (about one part in a thousand); after this they are again rinsed in pure water.
The Indian vat is much more easily managed than the foregoing; it presents less danger of failure, from the fact that it is quickly exhausted, and also from the fermentative process, which is so difficult to govern in the pastel vat; this vat not having time to change in character. It is prepared by first introducing an equal quantity of madder and of bran, and a triple quantity of potash; this is to be gradually heated until it reaches a temperature of 167° Fah., and we then add to it the indigo, thoroughly agitating the matters for half an hour. The vat is maintained at a temperature of 86° to 100° Fah., by keeping it closely covered, and at the same time the mixture is to be stirred occasionally at intervals of twelve hours. It should by this time present a beautiful green shade, the liquor being surmounted by a copper-colored pellicle and a purplish froth. We may now commence the dyeing, following the same course as with the pastel vat; but the stirrings being here repeated much more frequently than with the other mixture, we can dye a larger quantity of wool within a given time. When the vat ceases to give a brilliant blue, we must altogether renew it; if it be merely weakened, we add to it a small quantity of freshly prepared liquor containing a few pounds of potash, and a little less bran and madder. In giving the dark and the clear sky blues, we must be careful to employ a quantity of indigo proportioned to the color which we wish to obtain, or, better still, we may use the previously exhausted vat for the dark blue.
When exposed to the influence of the putrid fermentation, indigo is decomposed and loses its color. If rendered soluble, it obeys the impulse communicated to the azotized matters with which it is brought into contact, although, if 77 macerated in pure water at the ordinary temperature, it is itself decomposed with great difficulty.
The pastel and the woad are very prone to the putrid fermentation, by reason of the large quantity of azotized matters which they contain, as do all the cruciferæ; they require therefore considerable care in their employment.
When a vat is mounted, if the fermentation be allowed to continue unchecked, after the appearance of the blue froth and the other signs already indicated, the liquor will acquire a yellow color similar to that of beer; the froth will become white; it will give out a stale smell and lose its ammoniacal odor; after a few days it will turn whitish, and exhale a smell at first similar to that of putrefied animal substances; then it will acquire the odor of rotten eggs, and set free sulphuretted hydrogen. The lime in the pastel and the woad vats, and the tartar-lee and potash in the other mixtures, are used for the purpose of preventing these accidents.
Besides the oxygenated compound, which is formed by the combination of oxygen with the extractive matters of the plants held in digestion, there is a production of carbonic acid which saturates the alkaline lee, and forms a carbonate of lime in the pastel vat. We find this attached to the sides of the vat in such quantity that the inside of these vessels becomes incrusted with it to a considerable depth. It is this product which dyers call the tartar of the vat; it effervesces with acids, and gives on analysis carbonic acid, lime, and a few particles of indigo. In the potash vat the solubility of the carbonate of potash prevents its deposition; but it is very probable that we have even here a formation of some carbonated products, perhaps in part formed at the expense of the carbonic acid of the air.
The soluble extractive principle being the only matter which remains in solution in the bath with the indigo, the lime, &c., we have formed deposits which, varying both in their volume and in the greater or less facility with which they are precipitated during the various periods of fermentation, lead to a more or less considerable waste of time. If we plunge a piece of woollen tissue into a vat which has been recently stirred, it will acquire a dark color, and will be found covered with brown stains which are with difficulty removed. When the woad or paste vat has been stirred, it need be left two or three hours only before plunging in the stuff, at least during the early months of its working, inasmuch as the pastel, being but slightly divided and attenuated, is readily precipitated; but when, by reason of its extreme division, in consequence of repeated operations, it is thrown down with less facility, the dipping should not be performed oftener than three times in the day.
The Indian vat requires less time than the others; we may even dye with it an hour after stirring the mixture. The potash, being soluble, forms no precipitate; while the ligneous fibre of the madder and the pellicles of the bran become deposited with great facility. We can also dip with these vats much oftener than with those made by pastel or woad.
The distinction between pastel and woad is not very clear. Schutzenberger says: “Pastel, woad, and satis tinctoria is a plant of the family of the crucifera. It would seem, however, that the term pastel as used by the old French dyers is applied to the leaves of the woad which have been fermented, formed into paste, and afterwards into balls, and which contain much blue coloring matter. And the term woad as distinguished from pastel is applied to the unfermented plant.”
78
We have to thank that excellent practical magazine, “The American Chemist,” for the following notes on the sicknesses of the warm vat, by F. W. Kugler, translated from Reimann’s Färberzeintung:—
In the wool indigo vat, among the principal “sicknesses” is the blackening of the vat, or “sharpening.” This arises from the presence of too much lime. When “sharpened,” the liquor, instead of having a waxy yellow color with a dense blue film on its surface, has no film; while the liquor is a dark blackish-green, and on being stirred shows a gray or white scum on its surface, while it emits at the same time a pungent odor. If the vat is only slightly affected, it is sufficient to add some bran and madder and to let it stand over night. If it has not quite recovered by morning, it may be necessary to heat it up, agitate it, and let it stand for a couple of hours, after which perhaps the addition of a little lime will be necessary.
If the vat is much sharpened, it is recommended to sink in it a bag of bran, and leave it over night, when the fermentation will have restored the vat in a considerable degree; but it will be necessary to add lime cautiously and by degrees, to bring it to a proper state for working.
The theory of the souring of the vat is given. Butyric fermentation takes place under certain circumstances, butyric acid being formed; and hydrogen is set free, which reduces the indigo. The addition of lime makes the vat too strongly alkaline, and sets ammonia free, which gives the pungent odor of the soured (verschäften) vat. Simultaneously the lime with the white indigo forms a difficultly soluble compound, which settles, and thus interferes with the working of the vat. The excess of lime must be removed, which is accomplished by introducing bran, which causes a lactic fermentation; and the lactic acid neutralizes the excess of lime, and destroys the lime compound with indigo which had been formed. The lime may be neutralized by the use of mineral acids, but there is danger in that case of precipitating the indigo.
A second “sickness” is “becoming too sweet.” The symptoms are,—the blue veins and surface film disappear on stirring, the foam gives a rustling sound, the bath assumes a reddish-yellow color, blue goods placed in the bath lose their color, and the vat has an unpleasant odor.
The vat when “too sweet” needs to be brought to the regular temperature, and lime to be added cautiously until the vat is brought to its normal state. It is safer to add an excess of lime and “sour” the vat, and then bring it back according to the directions under that head, than to add too little, as less indigo is lost. To use up all the dye and to dye a light blue, as little lime should be present as is consistent with the workings of the vat.
The cause of the “falling away” of the vat is a too active fermentation, which produces considerable lactic acid, from which butyric acid forms, setting free hydrogen, thereby making white indigo, which, if the action is allowed to continue, changes to a compound from which the indigo cannot be recovered. If lime is added, the lactic and butyric acids unite with it and precipitate it, while the excess precipitates the white indigo, which is slowly recovered, as fermentation progresses, which forms lactic acid, which, taking the place of the white indigo, sets it free. Besides the sicknesses, there are various results of mismanagement, of 79 which the first is overwarming, which causes the bath to turn brown, which is the beginning of the souring.
When the bath begins to sour from overheating, some logwood should be added and then bran, and the vat left to itself over night. The reason of it is that the temperature is too high for the desired fermentation to operate. The vat sometimes suddenly turns green, and even when indigo and the other necessary ingredients are added it remains of this color. This is called the “breaking up of the vat.” The reason is that the temperature is too low; to remedy it, it is necessary to add logwood and bran, warm it up, and stir, when it should stand for some hours.